57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI750534-DNI7: Reversibility and Kinetic Trapping in Polymeric Nanostructures
Marina Tsianou, PhD, State University of New York at Buffalo
The goal of this research project is the fundamental understanding of the mechanisms behind phase separation, kinetic trapping, and reversibility in polymeric nanostructures that will in turn empower the rational formulation of such mixtures in a wide range of commercial applications where the efficiency and long-term stability of products play a crucial role. We focus on mixtures of polyelectrolytes with oppositely charged polymers or surfactants or particles. Such mixtures are typically employed in tandem for structuring aqueous media. The structuring is necessary for conferring viscoelastic rheological behavior (a polymer feature) to water, for improving colloidal stabilization, and for enabling a hydrophobic microenvironment (a surfactant feature) suitable for solubilization of molecules otherwise insoluble in water. The solution behavior of each of the components is important, however, the final performance of the formulated product depends to a large extent on the interplay of molecular interactions between the different species involved.
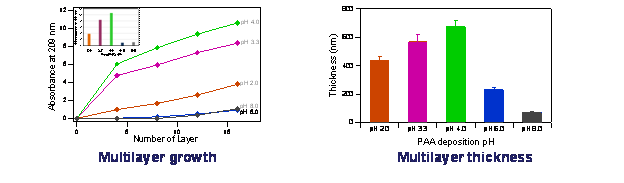
Previously we reported on interactions in systems involving positively charged poly(diallyldimethylammonium chloride), PDDA and oppositely charged poly(acrylic acid), PAA. We investigated the effect of pH on the PDDA/PAA complex formation in solution and compared the behavior to multilayers formed by subsequent deposition of PDDA and PAA (Layer-by-Layer assembly method). A schematic representation of the oppositely charged multilayer formation as opposed to the complex formation in solution is shown in Figure 1. Our results indicated pH-depended differences in the growth and thickness (obtained by AFM film evaluation at 14 layers) of the multilayers as shown in Figure 2. In addition, we have investigated the effects of the polymer molecular weight on the complex or multilayer formation and their stability and disassembly behavior when exposed to aqueous solutions of different pH values.
Figure 1. Oppositely charged multilayer (on surfaces) and complex (in aqueous solution) formation.
Figure 2. PDDA/PAA multilayer growth and film thickness at different pH values.
Separately, we are investigating the phase behavior of positively charged PDDA, cationic hydroxyethyl cellulose (cat-HEC), hydrophobically modified cationic hydroxyethyl cellulose (HM-HEC) when these are mixed with oppositely charged polymers or surfactants including poly(acrylic acid) (PAA), HM-poly(acrylic acid), poly(styrene sulfonate) (PSS) or sodium dodecyl sulfate (SDS) surfactant. We determine the phase behavior and stability of the non-equilibrium structures formed by these species, and assess how easily they can be switched over to other states (one-phase solutions or macroscopically phase-separated mixtures) by perturbations in the concentration of the components. We explore the effects of different mixing procedures on the stability of the samples. In addition, we have performed neutron scattering experiments on oppositely charged systems and we are currently in the process of analyzing the obtained results.
Plans for the upcoming year include studies on the time-evolution of complex formation and structure upon mixing of the ingredients, as affected by the molecular characteristics of the species, concentrations, and stoichiometric ratio. In particular, we are exploring the effects of pH, ionic strength of solution, molecular weight, and nature of polyelectrolyte (strong or weak) on the behavior of complexes formed in solution and we try to correlate this behavior with multilayer formation. We expect that our results will provide fundamental insights into the interactions of oppositely charged polymers and will directly impact several applications where formation and dissolution of complexes or control of deposition at surfaces and disassembly/erosion of multilayers play an important role.