57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND551448-ND5: Novel Mixed Metal Carbide Catalysts for Petroleum Processing
Friederike C. Jentoft, Dr. rer. nat., University of Oklahoma
Introduction
The goal of this research project is to prepare novel mixed metal carbides for applications in catalysis. Carbides currently constitute only a small group of catalysts, with a strong emphasis on simple carbides such as Mo2C or WxC (x=0,2). We aim to expand this class of catalysts by preparing mixed carbides, in which two metals are combined with carbon in a single phase. Issues in the preparation of carbides concern phase purity, surface alteration through oxide or carbon layers (often resulting in passivation), and surface area. Traditional methods of solid state synthetic chemistry typically require high temperatures, thus facilitating sintering and producing low surface area materials. In the first period of the project, various preparation methods for mixed carbides were tested.
Because of their high thermal stability, carbides can be used for processing under harsh conditions as required to convert heavy petroleum components such as polycondensed aromatic molecules. Our catalysts were tested for the hydrogenation and ring opening of toluene, which served as a model compound.
Preparation of mixed metal carbides
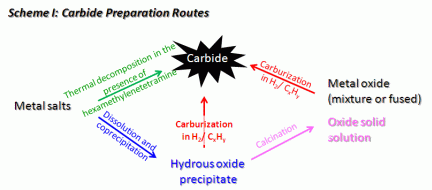
The preparation of carbides was sought by several methods, as illustrated in Scheme 1. The synthesis via decomposition of metal salts in the presence of hexamethylenetetramine, which serves as a reducing agent and as a carbon source, is a relatively new development (Chouzier et al., 2006). We succeeded in producing mixed carbides that had not been synthesized by this route before; specifically we produced Nb0.8W0.2C, a material with a known structure. However, it was difficult to obtain homogeneous materials that did not contain residual oxidic components or excess carbon, as shown by elemental analysis of the carbon content and by electron microscopy.
The preparation of simple and complex carbides through carburization of oxides in a H2/hydrocarbon atmosphere was also successful. To produce mixed carbides from oxides, two methods of mixing are reported in literature: (i) physical mixtures of two oxides, which provide a limited degree of mixing, or (ii) fused oxides, which require a high temperature synthesis and are bound to have low surface areas. We developed a new method, characterized by the production of a mixed oxide through calcination of a co-precipitated hydrous oxide, thus achieving an intimate mixture of both metal cations while applying only moderate temperatures. The subsequent carburization was monitored by thermogravimetry with online mass spectrometry, and the weight losses corresponded to the transformation of oxides into carbides.
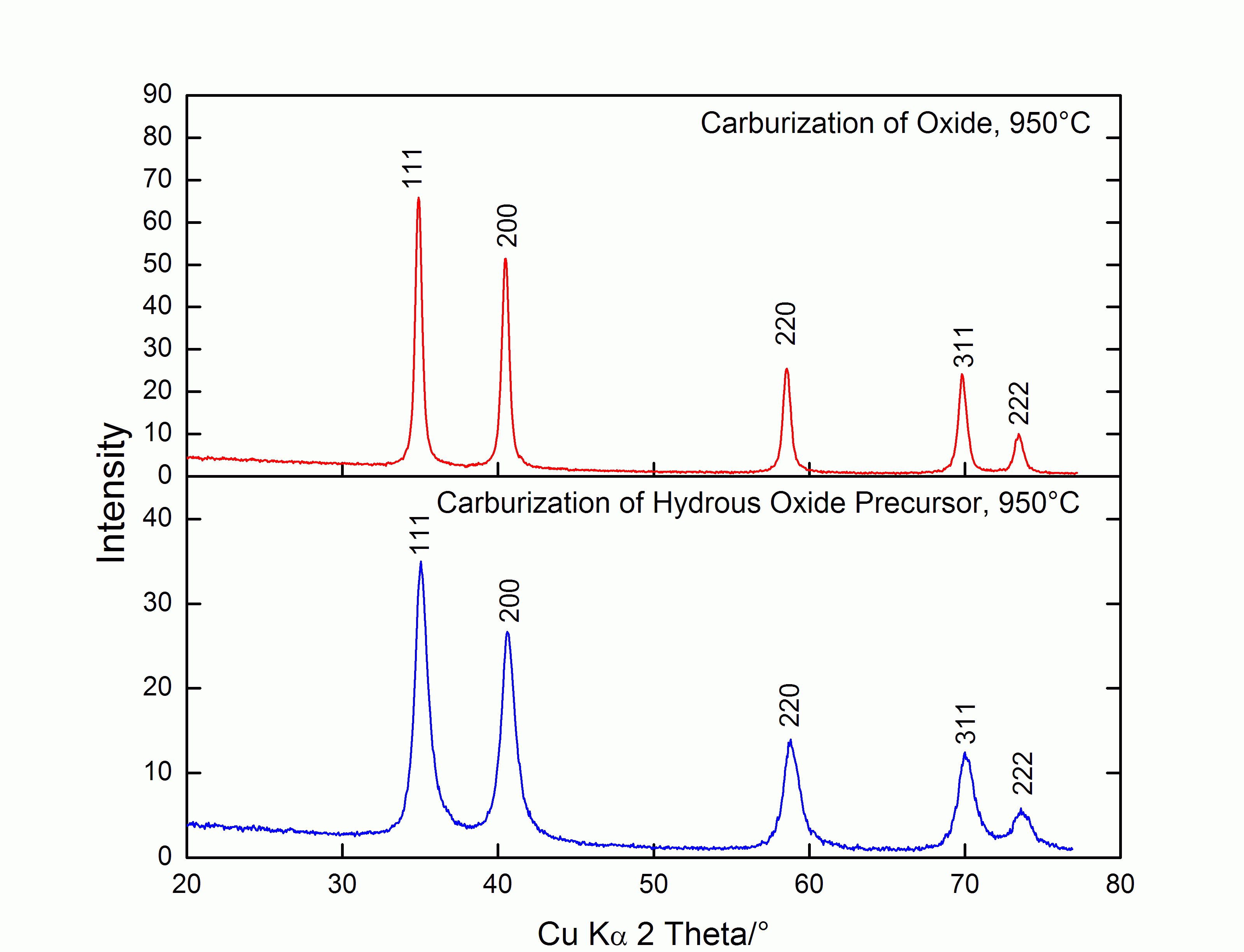
Figure 1: Powder diffractograms of Nb0.8W0.2C obtained via different routes (measured at room temperature).
It was even possible to obtain the carbide by direct carburization of the hydrous oxide precursor that was obtained in the co-precipitation, provided the carburization temperature was high enough. In Figure 1, the diffractogram of a carbide from a two-step synthesis involving co-precipitation and carburization is compared with the diffractogram of a carbide from a three-step synthesis involving co-precipitation, calcination, and carburization. The reflections in both diffractograms can be assigned to niobium tungsten carbide, ICDD PDF#01-071-6073. In this case, carburization was performed at 950°C; however, carbide formation was also observed at temperatures as low as 800°C (with intermediate calcination to oxide) or 850 °C (through direct carburization). It is hoped that low carburization temperatures will lead to materials with higher surface areas.
The two or three-step method involving co-precipitation, optional calcination and carburization is our current route of choice for the preparation of carbides with a broader range of compositions. Ongoing syntheses target carbides containing combinations of two metals in various ratios; at present, the selection of elements includes vanadium, niobium, molybdenum and tungsten.
The carburization agent was ethane, following literature reports of a higher H2 adsorption capacity when using this agent as compared to using methane or butane (Xiao et al., 2001). Ethane was also used to prepare a benchmark catalyst, Mo2C, from commercial MoO3. Investigations of the carburization kinetics by thermal analysis are under way; data on the solid state kinetics of carburization reactions are nearly absent in the literature and should be valuable for the optimization of the temperature program.
Catalytic activity of carbides in toluene hydrogenation and ring opening
The carbides were tested for toluene hydrogenation and ring opening at 30 bars of H2. The benchmark Mo2C started to become active for hydrogenation at 200 °C and produced significant amounts of methylcyclohexane at 250°C; ring opening products were observed at 300°C. Only mixed niobium tungsten carbides from the co-precipitation¾calcination¾carburization route have been tested so far; they did not exhibit any remarkable catalytic properties.
Summary and impacts on PI and students
In summary, various routes to the preparation of novel mixed carbides have been identified, and these routes should be suitable to prepare a wide variety of mixed metal carbides. Moreover, there is some promise that carbides can be obtained under comparatively mild conditions, which should result in higher surface area materials more suitable for catalytic applications. The project has allowed the PI to expand into a new field of research and currently supports one PhD student.