57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND350690-ND3: Dimetal Nitrido Chemistry
John Ferguson Berry, PhD, University of Wisconsin (Madison)
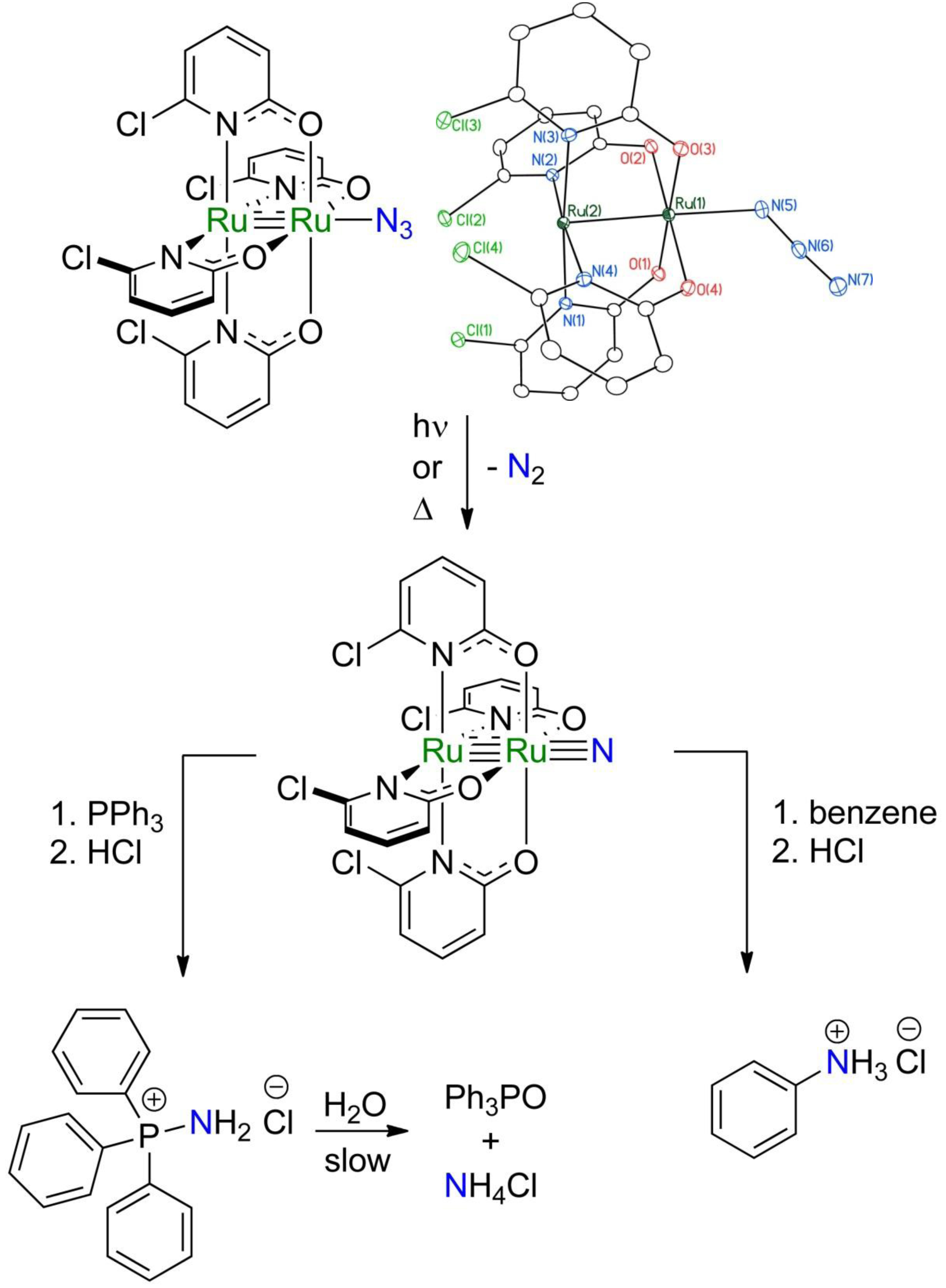
This grant supports research in the Berry lab on bimetallic nitrido complexes having a linear M–M≡N structure featuring a direct metal-metal bond. These complexes bear structural similarity to putative dirhodium nitrene intermediates that are implicated in catalytic C–H amination reactions. Our lab has recently observed intramolecular C–H amination to occur through the intermediacy of bimetallic nitrides, and one of the major goals of this grant is to expand this chemistry to intermolecular reactivity. To this end, we have synthesized the Ru2(chp)4N3 molecule, as shown below, which features no organic ligand moieties surrounding the azide group due to the arrangement of ligands around the metal. Thus, upon photolytic or thermolytic conversion of the azide to a nitride, no intramolecular C–H functionalization should take place and, instead, it should be possible to observe intermolecular reactivity.
To date, we have investigated reaction of the Ru2(chp)4N nitrido intermediate with two substrates: triphenylphosphine, and benzene. After photolysis of a solution of Ru2(chp)4N3 in the presence of ten equivalents of PPh3, and following subsequent workup with HCl gas, we have established by 31P NMR that Ph3PNH2Cl is formed in > 60 % yield. This product is proposed to result from reaction of the nitrido intermediate with PPh3 to form a Ru2–N=PPh3 complex, which then reacts with HCl to yield the Ru2(chp)4Cl starting complex (recovered in quantitative yield) and Ph3PNH2Cl. Thus, this reaction represents the first time we have been able to observe intermolecular reactivity with Ru2 nitrido complexes. There are two other noteworthy aspects of this reaction. First, since the Ru2(chp)4Cl starting material is recovered in quantitative yield from this process, we can close a synthetic cycle for converting PPh3 to phosphinimide using stoichiometric amounts of sodium azide as the N atom source. Moreover, the phosphinimide product, Ph3PNH2Cl, slowly hydrolyzes in water to produce phosphine oxide, Ph3PO, and ammonium ion. Thus, this synthetic cycle may be used to produce ammonia.
There are several outstanding questions that we would like to address regarding this phosphine chemistry. The yield of phosphinimide appears to be limited to ~ 60%, yet we obtain Ru2(chp)4Cl in 100% yield. Thus 40% of the nitride N atoms are unaccounted for. We have two hypotheses that we are exploring to account for this apparent mass imbalance. One possibility is that conversion from azide to nitride is less than quantitative. Thus, we plan to quantify nitride formation using EPR spin quantification techniques, since the nitride complex is known to show a very characteristic and easily interpretable EPR signal. Another possibility is that the nitride complex is formed, but undergoes bimolecular coupling to yield N2, and this bimolecular reaction competes with intermolecular N atom transfer to the Ph3P substrate. Another question we would like to address is whether or not it would be possible to generate the Ru2 nitride complexes from N2. To this end, we are exploring one electron reduction of Ru2(chp)4Cl in attempts to synthesize an N2 complex such as Ru2(chp)4(N2), which would represent the first step in converting N2 into a reactive Ru2 nitride.
We have also examined thermolysis and photolysis of Ru2(chp)4N3 in neat benzene to see if direct C–H amination of benzene to form aniline could be possible. Currently, aniline is formed from benzene in an energy-intensive two-step process involving first nitration of benzene in HNO3/H2SO4 at elevated temperatures, followed by reduction of nitrobenzene by molecular hydrogen using a Pt catalyst. Thus, a one-pot method would be quite useful. Thermolysis and photolysis of Ru2(chp)4N3 in neat benzene give identical spectral changes. After work-up with HCl, the Ru2(chp)4Cl starting material is recovered in quantitative yield, but we have had difficulties quantifying aniline. Early efforts using GC-MS measurements showed the presence of aniline, though we found that aniline sticks to the GC column that we were using, so we question those results. Newer experiments using 1H NMR to monitor the reaction progress have so far failed to detect any aniline. We don't fully understand this system, and there are some further experiments we need to do to test the feasibility of converting benzene to aniline. At the moment, we do not know the fate of the N atoms in this reaction, and would like to use 15N labeled azide to track them down.
We also have ongoing synthetic efforts to prepare the Os2 and Re2 analogs of the Ru2 nitride complexes described here. At the moment, we have identified some key starting materials, but have not yet been able to prepare the nitrido compounds. This will be a focus of our research in the coming year.