57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR449399-UR4: Demystifying Microwave Assistance in Homogeneous Catalysis: Chemical Kinetics of Microwave-Heated Palladium Reactions
Andrew W. Holland, PhD, Idaho State University
Many palladium catalyzed reactions are reported to enjoy dramatic acceleration when heated by a microwave rather than an oil bath, even at comparable temperatures and under otherwise similar reaction conditions. The cause of this improvement in many homogeneous systems is not understood, and the original goal of this project is to locate the origin of this effect by profiling reaction kinetics, rather than just measuring overall reaction yields, to distinguish differences in catalyst lifetime, induction periods, average rates, and other possible effects of microwave conditions.
In the first phases of this work, kinetic studies repeatedly showed that the actual differences between the two heating methods were much smaller than the reported reaction times indicated. This is not wholly surprising, as reports of yield and rate improvements by microwave heating were focused on improving outcomes rather than revisiting previous results. In our experiments, however, replication of optimal reported microwave conditions using an oil bath repeatedly produced kinetic traces similar to those of microwave reactions themselves.
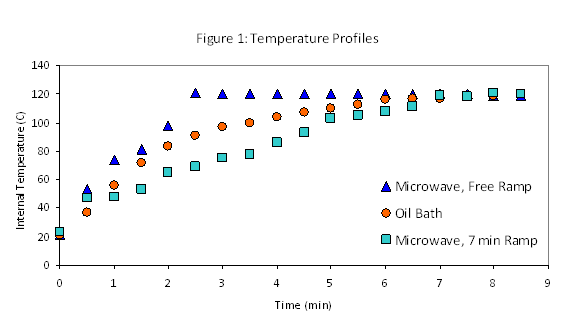
The results were not exactly the same, however. Particularly at early reaction times, modestly higher rates were frequently observed in microwave assisted reactions. In light of the growing body of evidence we had gathered against non-thermal microwave effects, we hypothesized that these differences might result from simple differences in the temperature profiles of different heating methods – although all the reactions under investigation ramped quickly to the target temperatures, even subtle variations of one or two minutes might explain the rate differences observed. This was investigated by carefully monitoring reaction temperature profiles of microwave and oil-bath reactions using our microwave's fiber optic temperature probe for both experiments. Although the ramp times were very similar, the microwave achieved the target temperature somewhat more abruptly, resulting in a significantly higher average temperature during the ramp time (Figure 1). This result led us to modify the microwave ramp time to better match the oil bath behavior, resulting in data from the two heating techniques that match more closely. However, it is worth noting that even after this adjustment, the actual shapes of the two temperature curves still differ. This is unavoidable with the microwave equipment at our disposal, and may still contribute to small differences between heating sources.
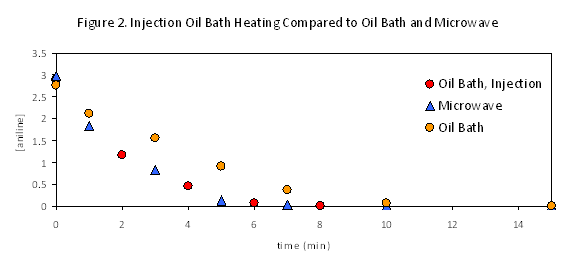
The fact that many of these reactions appeared to benefit simply from more rapid initial heating suggested that we might be able to emulate microwave effects by preheating reaction mixtures prior to catalyst injection. Injection heating has a long history and is widely used in certain fields of chemistry, but is less commonly applied in academic organic chemistry. This approach was applied to the previously studied reactions in detail, (eq 1, Figure 2) and to additional reactions reported to benefit from microwave acceleration (eq 2-3). In each case results similar to microwave heating were observed. These results show that in all those reactions investigated to date, the rate benefits of microwave heating can be attributed, after other variables are adequately controlled, to thermal effects rather than any special microwave effect. This means that they can be reproduced without a microwave, and argues for wider application of injection heating in cases where it would be more convenient than a microwave, and likewise for application of microwave heating to systems that have previously benefited from injection approaches.