57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND750941-ND7: Tetrazines for Hydrogen Storage
Douglas A. Loy, PhD, University of Arizona
Tetrazine Polymers for Hydrogen Storage
The goals of the first year were to synthesize the bis(dimethylpyrazole)tetrazine monomer (1), prepare polymers from 1 based on nucleophilic displacement of the dimethylpyrazole groups, and begin to study the hydrogenation/dehydrogenation chemistry of the tetrazine ring system. The solubility of tetrazines in different polymers was also evaluated to explore the possibility of hydrogen storage in solid solutions.
Monomer synthesis
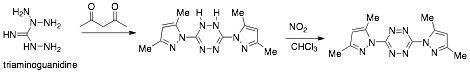
The monomer was synthesized by condensing triaminoguanidine with 2,4-penatandione to the dihydrotetrazine followed by oxidation to the desired tetrazine 1 (scheme 1). The dihydrotetrazine was observed to oxidize to 1 upon standing in air.
Scheme 1. Synthesis of tetrazine monomer 1
Tetrazine 1 and its derivatives were bright red or orange crystalline materials (Figure 1). The hydrogenated tetrazines were pale yellow (Figure 2).
Figure 1. 3,6-bis(3,5-dimethyl-1H-pyrazol-1-yl)-s-tetrazine (1)
Figure 2. 3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2-dihydro-1,2,4,5-tetrazine
Tetrazine hydrogenations
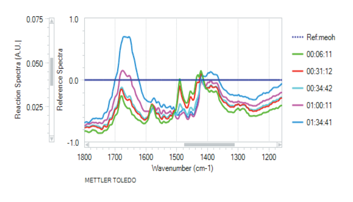
Samples of 1 and 3,6-diphenyl-1,2,4,5-tetrazine 2 were hydrogenated with one atmosphere of hydrogen in the presence of chloroplatinic acid. The progress of the hydrogenation could be readily followed with in-situ infrared spectroscopy (ReactIR, Figure 3) and by the bleaching of the red solution to a pale yellow. The IR spectrum shows a decrease in peaks at 1450 and 1490cm-1 and a growth in peaks located at 1380 and 1650cm-1 as the reaction proceeds. With introduction of oxygen, the hydrogenated products quickly oxidized back to the respective tetrazines indicating that the hydrogen can be recovered.
Figure 3: Shift in IR peaks as hydrogenation of 1 with increasing time.
Tetrazine solutions in polymers
It occurred to us during our investigations, that it may not be necessary to prepare polymers of tetrazines in order to effect solid state storage. If the tetrazines can be dissolved in polymers, particularly those with glass transition temperatures lower than room temperature, realtively simple hydrogen storage systems could be prepared more readily and potentially with greater quantitites of hydrogen. To pursue this line of investigation, we examined the solubility of the tetrazines in molten polymers and in polymers already dissolved in solvents known to dissolve tetrazines 1 or 2. Polydimethylsiloxane, a liquid polymer, would have been an ideal candidate that would allow rapid hydrogenations and could still be crosslinked to afford a solid storage media. However, while some tetrazines 1 and 2 were ssoluble at elevated temperatures, the materials crystallized out with cooling to room temperture. Better results were obtained with polystyrene (Tg 100° C), polyethylene oxide (Tg -35°C, Tm 51 °C, Figure 4.), and polybutadiene (Tg -80°C).
Figure 4: 3,6-diphenyl-1,2,4,5-tetrazine and chloroplatinic acid dissolved in polyethylene oxide
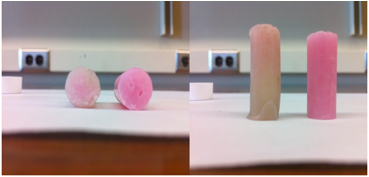
Polystyrene provided a glassy matrix for tetrazine 2 and Pd-C catalyst that showed not apparent decolorization upon exposure to hydrogen gas (Figure 5). Tetrazine 2 and Pd-C in polyethylene oxide, on the other hand, showed decolorization with exposure to hydrogen.
Figure 5: Hydrogenation of 2 in polyethylene oxide using chloroplatinic acid as the catalyst. The dark pink cylinder represents the control sample, and the light yellowish pink cylinder is a sample after hydrogenation. There is a distinct hydrogenation front that has penetrated about a third of the way into the cylindrical test sample in the photo on the left.
Polymerization experiments
Polymerization involves reaction of 1 with a co-monomer bearing two nucleophilic groups, such as amines or thiols. The dimethylpyrazole groups would be displaced by the new groups in a step growth polymerization. Reaction of the tetrazine with 1,2-diaminoethane or 1,6-diaminohexane resulted in insoluble materials due to multiple additions. We are now looking at co-monomers with secondary amine groups to prevent crosslinking reactions due to the amine groups along the linear polymer reacting with additions 1 (Scheme 2).
Scheme 2. Polymerization of tetrazine monomer 1 with diamine co-monomer
We have prepared a number of new tetrazine model compounds based on the reaction of simple amines with 1 (Figure 6). These studies have helped us understand the kinetics of the polymerization reaction. We also attempted to react 1 with ethane diothiol, but discovered that the reaction does not proceed. We will attempt to prepare the 3,6-dichlorotetrazine, that is known to react with thiols, from 1.
Figure 6: Tetrazine compounds synthesized by mono and disubstitution of pyrazole from 1 with propyl amine
 |