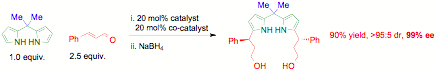57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151581-DNI1: Pyrrole-Based Organocatalysts for Chemical Rearrangements and Living Polymerizations
Catharine H. Larsen, PhD, University of California (Riverside)
Chemistsstrive to meld the discovery of reactions to build complex structures withenvironmentally friendly access to these synthetic targets. Methods that formbonds between multiple starting substrates reduce waste, time, and energyexpended. The fundamental properties of achiral dipyrromethanes and theirdipyrrin complexes are avidly studied due to their utility in catalysis,materials, biosensing, and energy storage. A method capable of introducingchirality to flank both pyrrole nitrogens could lead to the development of bothchiral organocatalysts and asymmetric ligands. With the support of this grant,our catalyst backbone was synthesized in >99% enantioselectivty, onediastereomer, and 90% yield. This first example of 1,9-chiral dipyrromethanesis accessed in 2 steps from pyrrole, acetone, and cinnamaldehyde. Ligands madefrom our chiral dipyrromethanes could be applied to induce enantioselectivityin our copper-catalyzed three-component couplings (3CC).
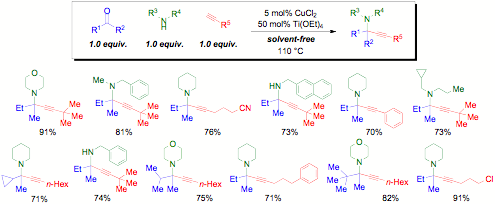
As part ofa program involving green multicomponent reactions that operate undersolvent-free conditions with 1 equivalent water as waste, we have developed thefirst catalytic ketone-amine-alkyne (KA2) coupling. Surprisingly,catalytic rather than stoichiometric amounts of Ti(OEt)4 weresufficient to access ketimine, paving the way for the addition of a copper(II)acetylide as the nucleophile. As the alkynylation of purified ketimine heatedin the presence of 5 mol% CuCl2 proceeds at half the rate of the 3CCin the presence of both Cu(II) and Ti(IV), the role of titanium appears toextend beyond acceleration of ketimine formation. This dual catalyst systemprovides a one-step synthesis of biologically relevant tetrasubstituted carbonsbearing amines and is appearing in Angewandte Chemie.
Cu(II)/Ti(IV)catalyst system would not have been developed without the support of this ACSPetroleum Research fund DNI award. Based on the dipyrromethane chemistry also supported by this ACS PRF grant, one female minoritygraduate student was awarded a dissertation year fellowship. This research hasserved as a training ground in organic methodology, organic and organometalliccatalysts, mechanistic analysis, and a variety of methods ofstructure-determination for 3 graduate students and 1 undergraduate (threefemale and one male). The next year of funding will support the preparation andpublication of additional related reactions. The project results and studenttraining served as a foundation for new group projects submitted to NSF forsupport. The PI has presented selected portions of this research during herseminar trips