57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151442-DNI1: Design and Development of Heterocycloaddition Reactions of Aza-Oxyallylcationic Intermediates for Chemical Synthesis
Christopher Jeffrey, PhD, University of Nevada, Reno
Introduction and background:
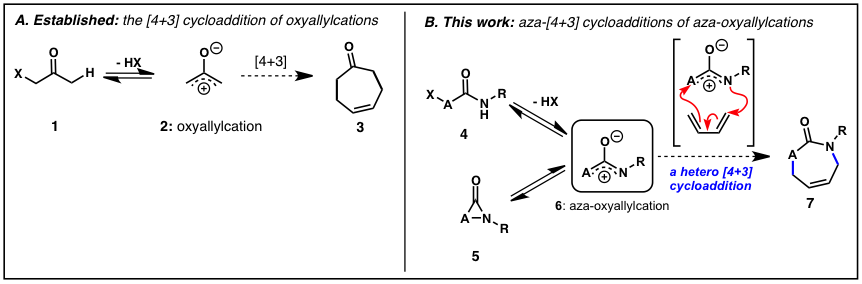
In old Irish folklore, the leprechaun is depicted as an elusive and mischievous dwarf, never seen, always avoiding capture, and hoarding a pot of gold at the end of a rainbow. Such was the case with the aza-oxyallylic cation 6, an elusive reaction intermediate that had teased chemists for decades and that quickly piqued the curiosity of our lab. The existence of the aza-oxyallyl cation 6 had long been suspected, but previous attempts at trapping the species had all been unsuccessful. Our group's proposal to intercept the aza-oxyallyl cation was largely inspired the work on oxyallyl cations 2 and their use in (4+3)-cycloaddition reactions with dienes to form seven-membered carbocycles 3. With the larger goal of developing new C-N bond-forming reaction methodology, we envisioned that the aza-oxyallyl cation 6 could be similarly employed to create seven-membered heterocycles 7 containing nitrogen and lead to development of alternative approaches to C-N bond formation.
Our early studies focused on the dehydrohalogenation of a-halo O-alkylhydroxamates 4 (X = halogen, R = O-alkyl). In these studies we found that aza-oxyallylic cations can be generated in-situ using basic trifluoroethanol (TFE) or hexafluroroisopropanol (HFIP) and that they react with cyclic dienes to afford the caprolactam 7 in good yield and diastereoselectivity. This was the first example that established definitive evidence of an aza-oxyallylic cation 6. Computational modeling of the uni-molecular ring opening of a-lactams 5 (R1 = Et vs. R1 = OMe) verified the stabilizing role of the N-alkoxy group. These results were published in March 2011 as a communication in JACS. We have extended this concept to the development of intramolecular cycloaddition reactions and the regioselective 1,4-diamination reaction of oxidized urea derivatives. Additionally, we have begun to pursue alternative methods of generating this intermediate as well as applying these reactions to the synthesis of biologically active target molecules. We have been invited write a manuscript that summarizes the evolution of our concept in a Synpacts article to be published this Summer and to publish a Synthesis review article on the historical developments related to the reactivity of aza-oxyallylic cations (expected Spring 2015). Below are some selected examples of the progress made in each project related to work that has not yet been published in the literature.
Scheme 1.
The development of 1,4-diamination reactions using N-chlorourea derivatives
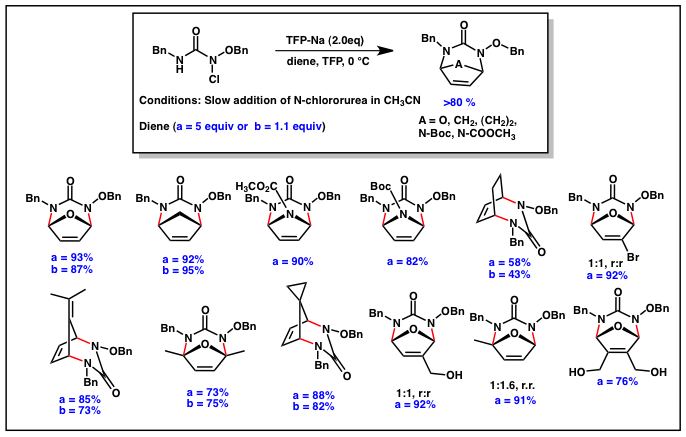
(1,n)-Diamines are ubiquitous functional groups found in numerous biologically active compounds and ligands. Despite the development of numerous methods of 1,2-diamination, only a few reports have been published that describe methods for the 1,4-diamination of conjugated dienes. The lack of examples of 1,4-diamination reactions inspired our group to pursue the development of a diaza-(4+3) reaction as a regioselective method for the 1,4-diamination of conjugated dienes (Scheme 2).
Our early results in this area, demonstrated that N-chloroureas could be dehydrohalogenated under basic conditions to the diaza-oxyallylic cation and that this intermediate reacts with cyclic dienes in a diaza-(4+3) reaction to afford bicyclic urea derivatives in good to high yield. It was found that the optimal conditions were to add the N-chlorourea over 3 hours to a solution of the basic 2,2,3,3-tetrafluoropropanol (TFP) and diene (1- 5 equiv). With this method in hand we explored the reactivity of this new heterocycle under a variety of different conditions. These results were reported this Fall in Organic Letters.
Scheme 2.
The development of 1,4-diamination reactions via a direct oxidation of symmetric urea derivatives
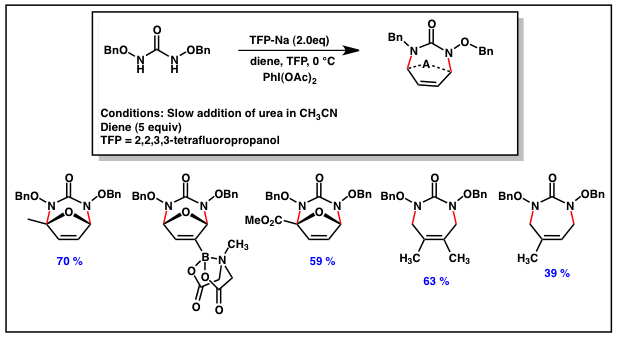
The diamination of dienes using N-chlorourea as the source of a diaza-allylic cation provided proof-of-concept results and verified that diazallylic cations are both viable intermediates and react with exclusive 1,4-regioselectivity with cyclic dienes (note the reaction of dimethylfulvene). However, failed attempts to circumvent the problems related to the lack of regioselectivity of the cycloaddition with unsymmetrical furans led us to explore an alternative method to generate the symmetric diaza-oxyallyl cationic intermediates. Hypervalent iodide reagents have been demonstrated to oxidize N-acyl-O-alkyl hydroxamates to their corresponding nitrenium ions. Given this data, we have recently pursued the development of an oxidative method for the generation of this intermediate. Our early results our highly promising, delivering the cyclic
Scheme 3.
urea derivative in good yield via the simple addition of the dibenzyloxy urea in CH3CN to a solution of diene PhI(OAc)2 in basic TFP (Scheme 3). Remarkably, these conditions have also enabled the reaction with acyclic (isoprene and 2,3-dimethylbutadiene) and electron-deficient furan derivatives (3-MIDA furan and Methyl-2-furanoate), dienes that failed to provide the desired cycloadduct under all other conditions. We are currently comprehensively evaluating the scope of this reaction and studying the enhanced reactivity of this system. These results will be published once the study has been completed.
The development of intramolecular aza-(4+3) cycloaddition reactions for the construction of polycyclic heterocycles and 2,5-substituted heterocyclic macrocycles
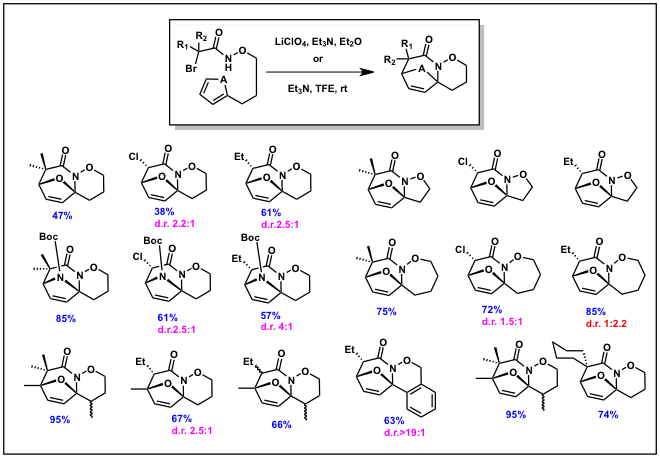
Polyheterocyclic scaffolds serve an important role in drug discovery and in the design and development of peptidomimetics. We rationalized that intramolecular aza-(4+3) cycloaddition reactions would provide a unique and powerful approach to the construction of such heterocycles and could extend the scope of reactivity of the aza-(4+3) cycloaddition reactions. Synthesis of a wide variety of cyclic diene substituted halo-hydroxamate derivatives and treatment to the Fšlish conditions provided the polyheterocyclic product in fair to good yield (Scheme 5).
Scheme 5.
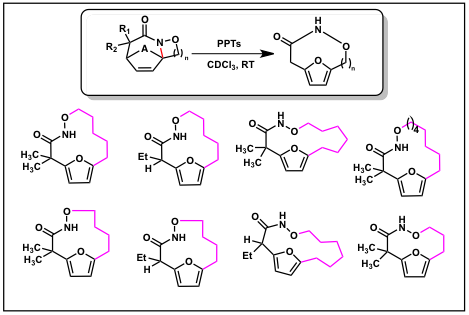
We have begun to pursue a study of the reactivity of highly functionalized scaffold and have found that the tricyclic furan derivatives efficiently undergo an acid-catalyzed ring opening to novel macrocyclic furan derivatives (Scheme 6).
Scheme 6.
The application of the aza-(4+3) cycloaddition reaction in target-directed synthesis
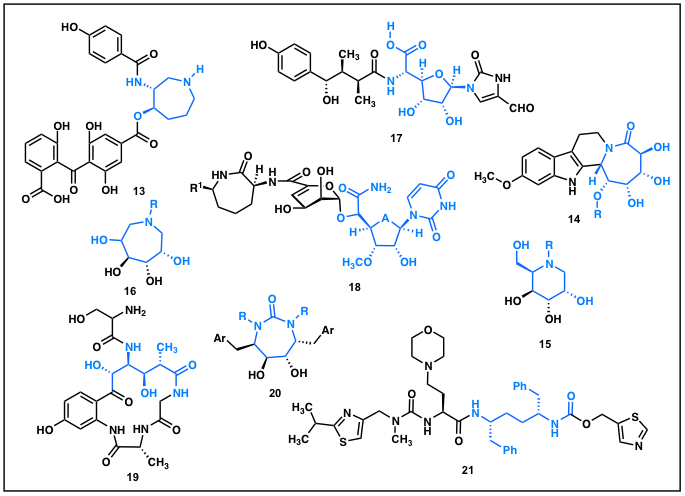
Our group has recently initiated studies that seek to employ our newly developed methods toward the total synthesis of a variety of target molecules. Many of our highly functionalized heterocycles map nicely onto an array of biologically active target molecules (Figure 1). Significant progress has been made toward the synthesis of balanol 13, bannisternoside A 14, and various amino glycosides 15 and 16.
Figure 1.