57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND149836-ND1: New Cycloaddition Reactions of Anionically Activated Dipoles
Daesung Lee, PhD, University of Illinois (Chicago)
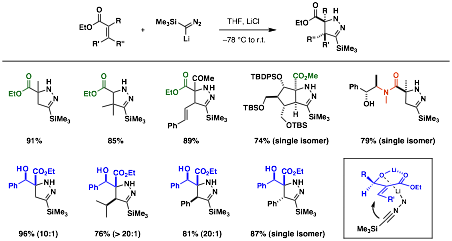
We have explored new cycloaddition chemistry of the anion of trimethylsilyldiazoalkane with a,b-unsaturated cyclic ketones as well as ketones with tethered alkenes and alkynes. This chemistry has been expanded beyond the a,b-unsaturated cyclic ketones. In addition to the previously reported results, we have developed a general protocol to form pyrazolines from a,b-unsaturated esters. Under optimized conditions, very high diastereoselectivity of products could be achieved. The high diastereoselectivity is probably is the consequence of the chelate formation that can discriminate the two diastereotopic faces effectively for the addition of the incoming dipole (Table 1).
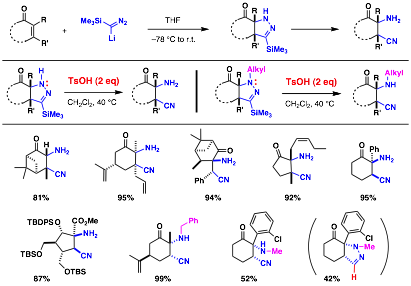
a-Amino ketone is a structural motif that exists in numerous natural products and pharmaceuticals. Conceptually, this functional group can be installed by a-amination, a process wherein a nitrogen-based electrophile reacts with an enolate nucleophile derived from carbonyl compounds. Many a-amination methods have been developed, yet most of these methods share a common problem dealing with removal of the extra functionality on the nitrogen after the C–N bond formation. Therefore, inventing new methods that can introduce a free amino group would have a significant merit over the existing a-amination methods.
Toward this goal, we envisioned the addition of lithium trimethylsilyldiazomethane to a,b-unsaturated ketones esters followed by mild N–N bond cleavage would lead to an efficient synthesis of a-amino ketone and ester. We have explored the N–N bond cleavage and postulated that the presence of a trimethylsilyl group on our pyrazolines would facilitate both an oxidative or proteolytic N–N bond cleavage (Table 2). We hypothesized that under oxidative conditions, a single electron transfer from sp3-hybridized nitrogen to an oxidant would generate a radical cation intermediate, which then will submit to an attack by a nucleophile at the trimethylsilyl group to induce a simultaneous C–SiMe3 and N–N bond cleavage, providing a-amino ketones or esters. In the other scenario, good to excellent yield of N–N bond cleavage product were obtained.
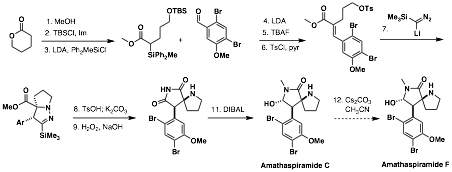
On the basis of these concatenated transformations involving pyrazoline formation and its N–N bond cleavage, we are able to construct molecular structures that contain a-amino quaternary centers. We applied the prowess of this transformation to the total synthesis natural products that have these structural characteristics such as amathaspiramides as shown in the (Scheme 1). Via standard transformations, the dipolar addition substrate was synthesized and the pyrazoline was obtained as expected. Cleavage of the N–N bond under the developed conditions provided the pivotal intermediate in a very short synthetic sequence. The elaboration of the nitrile functionality to the corresponding diimide followed by regioselective reduction provided amathaspiramide C, which will be transformed to amathaspiramide C under the known conditions.