57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI950576-DNI9: Linking Ion Structure with Aromatic Solubility in Ionic Liquids
Wesley A. Henderson, PhD, North Carolina State University
During the first year of the grant, the solubility and recrystallization kinetics of polyaromatic compounds (PACs) in ionic liquids (ILs) was explored for two classes of PACs with and without heteroatoms. The structure of the IL cations was found to be highly relevant in determining the solubility of the different PACS, as was the structure of the PACs.
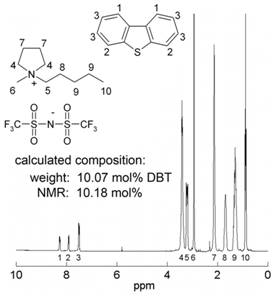
Work in the second year focused on revising and tuning some of the previous work as part of the preparation of a summative manuscript on the work. As recrystallization studies were used, it was found that variable results could be obtained due to crystallization kinetics which were sensitive to a range of factors. Therefore, it was necessary to repeat some of the measurements numerous times to obtain a high confidence in the results. To validate the recrystallization studies, the NMR characterization work initiated in the previous year was expanded upon. The PACs were dissolved in the ILs by heating/stirring at elevated temperature and then the sample vials were stored at ambient temperature. If the solubility limit was exceeded, crystals formed and grew in the mixtures until equilibrium was achieved. The liquid portion of the samples (i.e., ILs saturated with dissolved PACs) were then extracted for analysis. 1H-NMR was used to determine the PAC solubility. To verify the accuracy of this method, the PY15TFSI-10 mol% DBT mixture was initially analyzed. No crystallization occurred for this sample over a 2 wk period. Excellent agreement was found between the determined NMR value for the DBF content and the value obtained from the mass of the materials used in the sample preparation (Fig. 1). Table 1 reports the obtained DBF solubility values for the samples for which crystals formed after 2 wks (if no crystals formed, a value of "20+" was included in the table). Excellent agreement was found between the NMR solubility data and the recrystallization time results previously reported.
Recrystallization is a key separation/purification methods for many materials. Many of the PACs which are commercially available are sold in low purity (i.e., 98%). A tremendous difference in pricing is found between this purity and the same materials with higher (i.e., ≥99%) purity. This is likely due to the need to purify these material with costly methods due to the low solubility of many PACs. The very high solubility found for the PACs in some of the ILs (Fig. 2), however, suggests that recrystallization is likely a facile, low-cost methods for achieving high purity. Although the ILs are not inexpensive, they can readily be recovered and continuously reused. Extensive work was thus performed to confirm this purification methodology.
The solubility of organic semiconductors (PACs)—including sexithiophene (Fig. 3) and rubrene—was also examined to determine if the ILs were useful media for processing organic semiconductors. These have extremely poor solubility in common solvents. They were found to have a rather limited solubility in the ILs, but enough can be dissolved (Fig. 3) to potentially be useful for distributing the semiconductor materials on devices. Methods were explored to deposit the materials through the addition of solvents (nonsolvents) to the mixtures in various ways to determine how the organic semiconductors are deposited and how this can be controlled.
Overall, this projects demonstrated ILs to be very effective solvents for a wide range of PACs. Further, the solubility can be tuned in a systematic fashion by modifying features of the IL ions (cation head group, alkyl chain length, inclusion of an oxygen atom in the alkyl chain or ring, etc.). The ability to then precipitate the PACs from the ILs through the additional of a nonsolvent (which can then be readily removed from the nonvolatile ILs by heating) makes ILs very effective for processing PACs.
Figure 1. 1H-NMR spectrum of PY15TFSI-10 mol% DBT. Calculated and NMR compositions were determined from the weight of the components during sample preparation and integrated peak intensities, respectively.
Table 1. Solubility (mol%) of DBF in ILs as determined by 1H-NMR (R is the cation alkyl chain length)
R = | 1 | 2 | 3 | 4 | 5 | 6 |
dibenzofuran (DBF)
|
|
|
|
|
| |
IM10RTFSI | 3.8 | 9.1 | 10.7 | 14.2 | 17.4 | 20+ |
PY1RTFSI |
|
| 11.3 | 14.1 | 17.2 | 20+ |
PI1RTFSI |
|
| 15.3 | 17.9 | 20+ | 20+ |
Figure 2. Sample vials at 22°C from crystallization measurements: PY1RTFSI-20 mol% PYR (a) 30 min and (b) 2 h after heating and PI1RTFSI-20 mol% PYR (c) 2 h after heating. From left to right R = 3, 4, 5 and 6 (the yellow discoloration originates from impurities in the PYR).
Figure 3. Dissolution of 1 mg of sexithiophene in 1.5 mL of IM116TFSI: (left) before) heating and (right) after heating.