57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND550780-ND5: Targeted Delivery of Organometallic Complexes to Low-Coordination Sites on Oxide Surfaces
Lisa McElwee-White, University of Florida
Jason F. Weaver, University of Florida
Heterogeneous catalysts will play a central role in developing technology that can meet global energy needs, including the production and utilization of chemical fuels. Unfortunately, conventional methods for synthesizing oxide-supported metal catalysts suffer from limited control over the structure of the active metal species. Our proposed catalyst synthesis method involves selective adsorption of organometallic complexes onto low-coordination sites of oxide surfaces, followed by removal of the ligands using non-thermal oxygen and hydrogen plasmas at temperatures low enough to preserve the original binding sites of the metal species. The hypothesis is that alcohols and amines will exhibit a preference for binding and reacting at low-coordination step and kink sites of TiO2 and SrTiO3 surfaces and that this can be used to guide the placement of metal atoms on the oxide support.
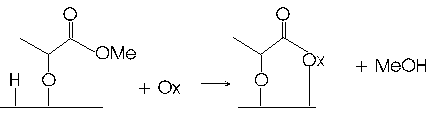
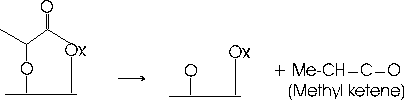
We have investigated structural properties of SrTiO3 (STO) surfaces as well as the decomposition of methyl lactate enantiomers on the STO(621) surface during this project. Our experiments with methyl lactate provide support for our hypothesis that the low coordination, kink sites of the oxide surface promote distinct chemical transformations compared with terrace sites. Temperature programmed reaction spectroscopy (TPRS) measurements demonstrate that methyl lactate initial reacts on STO(621) through deprotonation of the OH group at temperatures near 200 K. Subsequently, the adsorbed moiety reacts with surface-bound OH groups to produce methanol over a broad temperature range from ~200 to 600 K. Figure 1 shows a representation of the proposed adsorbed species that is produced by de-esterification of methyl lactate on STO(621). The symbol "Ox" represents an oxygen atom of the oxide surface. Above about 450 K, the organic adsorbate decomposes further to liberate methyl ketene (Figure 2), with a fraction of this product desorbing and the remainder decomposing to ethylene, water and CO2.
Figure 1
Figure 2
The TPRS data show that the methyl ketene desorption peaks reach maxima at 610 and 625 K for decomposition of the R and S ML enantiomers, respectively, independent of the initial methyl lactate coverage. In contrast, desorption peaks of other products do not exhibit such a systematic difference between the two enantiomers. The peak temperature difference for the methyl ketene product indeed suggests that the pathway for methyl ketene formation is enantiospecific. The reaction scheme discussed above further shows that a chiral intermediate is likely to result from methanol formation and can undergo direct C-O bond cleavage to generate the methyl ketene product. The implication is that the kink sites of STO(621) are enantiospecific toward methyl ketene production, giving rise to a peak temperature difference of 15 K for decomposition of the R vs. S enantiomers. The apparent enantiospecificity of methyl ketene production is quite interesting, and provides evidence that the kink sites of STO surfaces can promote distinct chemical transformations compared with the terrace sites.
We have also devoted considerable effort to preparing and characterizing (12 2 1)-oriented STO samples. The (12 2 1) and (621) STO surfaces have structurally-identical kink-step sites, but the terraces of the (12 2 1) surface are twice as wide as (621). The aim of changing to the (12 2 1) surface is to minimize surface restructuring and thereby obtain flatter surfaces that are more amenable to atomically resolved STM imaging. The new student working on this project cut and polished several STO(12 2 1) samples, designed and built a new sample holder to avoid sample cracking, and conducted extensive sample annealing in UHV to render the STO samples sufficiently conductive for characterization using low-energy electron diffraction (LEED). After prolonged sample reduction, we have successfully collected LEED patterns of the STO samples which are consistent with the anticipated (12 2 1) surface orientation. We are planning to further characterize the structure of the STO(12 2 1) sample and examine the surface reactivity toward OH-functionalized Ru compounds in the near future.
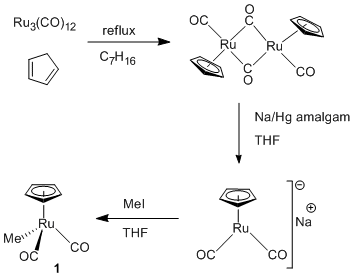
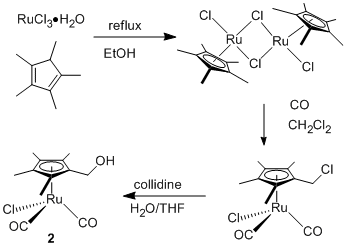
In parallel with studies of reactions of the hydroxyl-substituted substrate methyl lactate on the oxide surface, we have been synthesizing metal complexes for deposition of catalytic sites. We first prepared CpRu(CO)CH3 (1, Figure 3), a complex with no site directing hydroxyl substituent for use as a model compound in control experiments. Complex 1 is a known chemical vapor deposition precursor for Ru films and will be used to optimize conditions for depositing organometallic species on the oxide surface. The hydroxyl-substituted cyclopentadienyl ring in compound 2 is the site-directing group for experiments targeting placement of catalytic sites by tethering the complex to the surface through the alcohol moiety. Complex 2 was synthesized as shown in Figure 4. An additional hydroxyl substituted complex (3) features a different ligand set than 1 and 2. Complex 3 is a Pd complex whose hydroxyl group is bound to a bipyridine ligand and was prepared via the route in Figure 5. Following the initial experiments with deposition of catalytic sites on the oxide surface using 2 and 3, we will prepare additional precursors designed on the basis of the initial results.
Figure 3
Figure 4
Figure 5