57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND150983-ND1: Exploring Indolidene Chemistry: New Opportunities for Complex Indole Synthesis
Ken Feldman, Pennsylvania State University
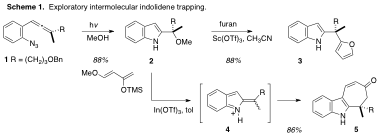
The ACSPRF/ND grant funded exploratory studies on the use of indolidene intermediates in alkaloid synthesis. This research proceeded along two distinct thrusts: (1) a survey of nucleophiles to determine which species were reactive enough to combine productively with the electrophilic indolidene intermediates, and (2) application of indolidene chemistry to the total synthesis of the alkaloid (–)-gilbertine (27). On the former topic (Scheme 1), preliminary experiments with transient intermediate indolidene 4, prepared via our recently described allenyl azide photocyclization sequence from 1, documented that high-yielding adduct formation is possible. Both furan (–> 3) and Danishefsky's diene (–> 5) were effective in forging new C-C bonds to the presumed intermediate indolidenium ion 4. The tricyclic species 5 bears some resemblance to the ambiguine family of marine alkaloids.
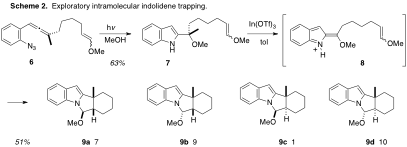
In a second series of experiments (Scheme 2), an intramolecular version of indolidene trapping was explored with 6. Exposure of the derived activated indole 7 to mild Lewis acid furnished the suite of C–C/C-N bonded adducts 9a-9d, in the ratio shown. If better stereochemical control with more biased systems can be achieved, a new and very efficient approach to members of the terpene-alkaloids can be envisioned.
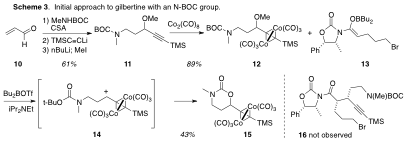
The initial attempt at gilbertine synthesis utilized a BOC protecting group (Scheme 3), but apparently the electrophile 14 generated by Lewis acid treatment of 12 engaged the proximate BOC group rather than the boron enolate 13 as desired, and cyclic carbamate 15 resulted; none of the desired alkylation product 16 was observed.
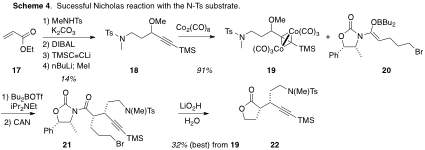
Switching the interfering BOC group for a tosyl group solved this problem (Scheme 4). Thus, the Nicholas reaction of 12 and 13 proceeded to give the alkylation product 21, which was typically processed on to the lactone 22 as a crude material. The overall yield for this sequence never exceeded 32%, and it appeared that the problem was not the Nicholas alkylation, but rather the subsequent lactone formation.
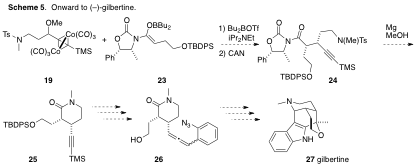
To circumvent this low yield, a modified boron enolate 23 will be prepared (Scheme 5). Its reaction with 19 (already in hand) should provide an alkylation product 24 that can be converted directly to pivotal lactam intermediate 25 without the need to form the challenging lactone. A key end-game intermediate allenyl azide 26 that links lactam 25 to gilbertine (27) is shown for convenience.
Work during the 2nd year of support from the ACSPRF will focus on (1) examining other carbon nucleophiles in intermolecular additions to indolidene 4, and (2) work towards (and hopefully completion of) the synthesis of (–)-gilbertine.