57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR149441-UR1: Gold(I)-Catalyzed Aromatic Claisen Rearrangements
James R. Vyvyan, PhD, Western Washington University
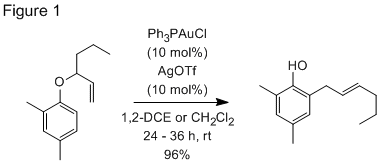
Much of our effort in year three of the project was a continued investigation of gold nanoparticle formation during the reaction of allyl aryl ethers in the presence of Ph3PAuOTf. (Fig 1) Particle size as a function of reaction time was studied with AFM and DLS measurements. It was shown that gold particles form before significant turnover of substrate is observed by 1H NMR and GC analysis. GC/MS analysis of the reaction mixture showed none of the organic compounds in the starting mixture were oxidized during the rearrangement reaction, leading us to conclude that the gold(0) particles form through a disproportionation of the gold(I) complexes in solution (Eq 1).
Equation 1: 3 Ph3PAu(I)OTf → 2 Au(0) + Ph3PAu(III)(OTf)3 + 2 PPh3
Analysis of the particle film with XPS indicated the presence of some higher oxidation states of gold present in the film, further supporting our conclusion. Based on these findings, we believe that Au(III) is responsible for the dominant rearrangement process, in agreement with the report of He's group that the rearrangement of allyl aryl ethers is faster with Au(III) than Au(I).
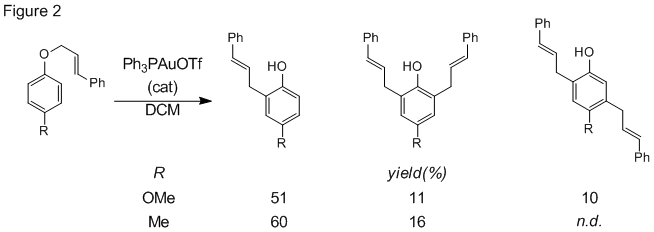
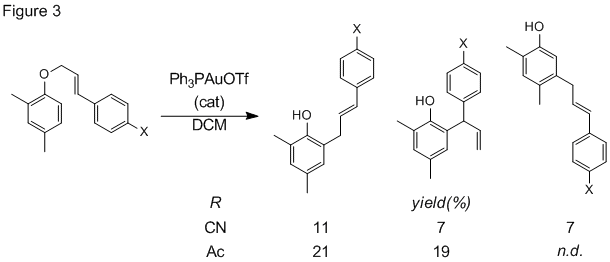
We also continued study of the reaction scope this year, specifically the effect of aryl substituents on the reaction of cinnamyl aryl ethers. Electron releasing groups on the aryl ring lead to good yields of rearranged products, including some dialkylated phenol products (Fig 2). Electron withdrawing groups on the cinnamyl ring, however, produced low yields of rearrangement products as a mixture of linear and branched isomers (Fig 3). These results are consistent with our previous data supporting an ionic mechanism.