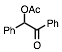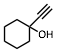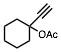57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR151036-UR1: Applications of Iron (III) Compounds as Catalysts in Organic Synthesis
Ram S. Mohan, Illinois Wesleyan University
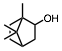
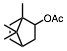
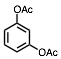
The conversion of alcohols to esters is an important synthetic transformation that has received considerable attention. Conversion of an alcohol to the corresponding acetate is typically carried out using acetic anhydride or acetyl chloride in the presence of pyridine or triethylamine as a catalyst. 4-(Dimethylamino)pyridine (DMAP) is known to cause a remarkable rate acceleration in this reaction. One problem with tertiary amines is that they are corrosive, toxic, and often highly unpleasant to work with. Lewis acids have also been reported to catalyze the acetylation of alcohols. These include Bi(OTf)3, Sc(OTf)3,, CoCl2, and I2. Many of these catalysts are either corrosive (such as I2) or very expensive (scandium salts). With increasing environmental concerns, it is imperative that new "environmentally friendly" reagents be developed. Our continued interest in developing environmentally friendly synthetic methodology prompted us to investigate a mild and catalytic method for the acylation of alcohols, phenols, and diols utilizing inexpensive, commercially available reagents. Herein we wish to report that iron(III) tosylate is a mild catalyst for the acylation of a variety of alcohols, phenols, and diols (Table 1). As can be seen from Table 1, the reaction worked well with 1° and 2° alcohols (entries 1-11), and phenols (entries 15, 16, 17 and 20). When acetic anhydride was used as the acylating agent the reaction could be carried out under solvent-free conditions. With allylic alcohols (entries 3 and 5), the use of solvent (CH3CN) gave fewer side products. In most cases, the crude product was found to be ³ 98% pure by 1H and 13C NMR spectroscopy and further purification was deemed unnecessary. For solubility reasons, CH2Cl2 was used as a solvent in case of benzil (entry 11). Although the methodology was not broadly applicable to tertiary alcohols, we were able to successfully acetylate some 3° alcohols. For example, 1-ethynylcyclohexanol (entry 12) gave a moderate yield of the corresponding acetate. When 1-methylcyclohexanol (entry 13) was subjected to the reaction conditions (in CH3CN), the crude product although colored was found to contain mostly (80%) the 3° acetate. However, chromatography yielded the pure acetate in only a low yield (38%).
Table 1. Iron(III) tosylate catalyzed acylation of alcohols, phenols and diols.
Entry | Alcohol | <>Anhydride R1 | | | Product | Yield (%)c | |||
1a | CH3 | – | 10 min | 94 | |||||
1b | Phd | CH3CN | 24 h, 70 °C | 73e | |||||
1c | n-Pr | – | 1 h | 85 | |||||
2 | CH3 | – | 1 h, 0 °C | 92 | |||||
3 | CH3 | – | 15 min, 0 °C | 95f | |||||
4a | CH3 | – | 25 min, 0 °C | 66e
| |||||
4b | Phd | CH3CN | 27 h, 70 °C | 66e | |||||
5 | CH3d | CH3CN | 22.5 h, 0 °C | 82g | |||||
6 | p-NO2C6H4CH2OH | CH3 | CH3CN | 45 min | p-NO2C6H4CH2OAc | 98 | |||
7 | CH3 | CH3CN | 10 min | 92 | |||||
8 | CH3 | CH3CN | 1 h 50 min | 93 | |||||
9 | Phd | CH3CN | 44 h, 70°C | 61e | |||||
10 | CH3 | CH3CN | 30 h, 50°C | 92 | |||||
11 | CH3 | CH2Cl2 | 18 h | 98 | |||||
12 | CH3 | CH3CN | 5 h, 0 °C | 95 | |||||
13 | CH3 | CH3CN | 21 h, 0 °C to rt | 38e | |||||
14 | Ph3COH | CH3 | CH3CN | 49 h, rt to 70°C | NRh | ||||
15 | CH3 | – | 2 h | 77 | |||||
16 | CH3 | CH3CN | 24.5 h, 50 °C | 95 | |||||
17 | CH3 | CH3CN | 1.5 h | 70,e | |||||
18 | CH3i | CH3CN | 3 h | 84 | |||||
19 20 | CH3i CH3i | CH3CN CH3CN | 50 min 50 min | 89 99 | |||||
21 | CH3j | – | 2 h, 0 °C | 96i, j | |||||
aReagent grade acetonitrile was used.
bAll reactions were run at room temperature unless otherwise mentioned, and reaction progress was monitored by GC or TLC.
cRefers to yield of isolated product that was deemed to be sufficiently pure (> 98%) by 1H & 13C NMR spectroscopy, unless otherwise mentioned. All products have been previously reported in the literature or are commercially available. The superscript next to yield refers to literature reference for spectral data of the product.
dReaction was carried out using 5.0 mol % catalyst.
eYield of product after purification by flash chromatography.
fProduct was determined to be 96% pure by GC
gReaction was carried out using 0.5 mol % catalyst.
hNo reaction was observed even when the mixture was heated at reflux for 29 h.
iReaction was carried out with 2.6 equivalents of acetic anhydride.
jProduct is commercially available (CAS # 6963-44-6).
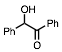
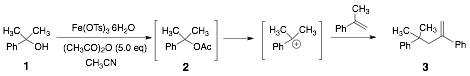
Any 1-methylcyclohexene that may have formed is likely to have been lost during removal of the solvent on a rotary evaporator, and hence was not seen in the 1H spectrum of the crude product. The hindered 3° alcohol, triphenylmethanol (entry 14), failed to yield the acetate even under reflux conditions, and the starting material was recovered unchanged. When 2-phenyl-2-propanol 1 (scheme 1) was subjected to the reaction conditions, none of the corresponding acetate was isolated. GC analysis of the crude product, which was obtained as a dark red-brown liquid showed that it mostly (88%) contained product 3. However, product 3 was isolated only in 26% yield after chromatographic purification of the crude product. Its identity was confirmed by 1H and 13C NMR spectroscopy, and HRMS. Based on the fact that the same product was obtained in the absence of acetic anhydride but at a much slower rate, we propose that product 3 arises via the initially formed acetate 2, which subsequently eliminates and dimerizes via a 3° carbocation.
Scheme 1
Attempts to make the monoacetate from a symmetrical diol 1, 5-pentanediol (entry 21) using one equivalent of acetic anhydride were not successful. When 1,5-pentanediol was reacted with 1.05 equivalents on acetic anhydride, the product was a mixture of the monocetate, diacetate and unreacted starting material. However, formation of the diacetate proceeded smoothly in the presence of 2.6 equivalents of acetic anhydride. We have previously reported that aliphatic TBDMS groups can be cleaved with iron(III) tosylate in the presence of a phenolic TBDMS ether. Consistent with this observation is the fact that we were able to acetylate a phenol in the presence of a phenolic TBDMS group (entry 17).