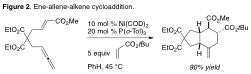57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150245-DNI1: Transition Metal Catalyzed [2+2+2] Cycloadditions for Carbocycle Synthesis
Erik J. Alexanian, PhD, University of North Carolina (Chapel Hill)
An important goal of chemical synthesis is the development of new transformations enabling the rapid construction of valuable complex architectures from simple building blocks. Our group has developed a number of complexity-generating multicomponent [2+2+2] cycloadditions that furnish stereochemically complex bicyclic carbocycles (e.g., hydrindanes and decalins) in a single step. These transformations use simple unsaturated components such as alkenes and allenes as substrate, and proceed with high levels of reaction chemo-, regio-, and stereoselectivity. We are also developing highly enantioselective variants of the [2+2+2] cycloadditions.
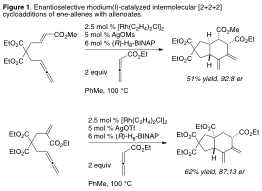
Over the past year, a major focus of our research has been the development an enantioselective variant of our rhodium-catalyzed [2+2+2] cycloaddition of ene-allenes with allene p-systems (Figure 1). Our efforts have culminated in an intermolecular enantioselective [2+2+2] cycloaddition of ene-allenes and allenoates for the synthesis of enantioenriched complex carbocycles possessing multiple stereocenters. This reaction represents a rare example of an intermolecular, multicomponent cycloaddition affording carbocyclic products containing multiple stereogenic centers. The reaction generates three bonds, two carbocyclic rings, and up to four contiguous stereocenters in a single step with excellent levels of reaction regio-, chemo-, and diastereoselectivity. The use of a catalytic system comprised of a rhodium(I) precatalyst, a silver(I) salt, and a bidentate phosphine ligand ((R)-H8-BINAP) delivers the carbocyclic products with good levels of enantioselectivity. Our mechanistic studies are consistent with a mechanism involving an unprecedented enantioselective allene-allenoate oxidative coupling, followed by incorporation of the alkene component.
We have also developed a general, intermolecular [2+2+2] of ene-allenes and a variety of diverse alkene components for the stereoselective preparation of complex cyclohexanoids (Figure 2). In this process, nickel-based catalysts facilitated the cycloaddition with greater efficiency than our rhodium systems. These cycloadditions involving multiple alkene components also proceed with high levels of stereoselectivity. Interestingly, simple modification of the catalyst system alters the reaction pathway to afford a number of other useful reactions involving the ene-allene substrate.
Catalytic [2+2+2] cycloadditions that are capable of forging multiple bonds and sp3 stereocenters are rare, despite the impressive synthetic potential of such processes. These complexity-generating reactions construct important classes of fused carbocycles with levels of efficiency unmatched by current synthetic protocols, providing fundamentally new strategic choices for the rapid construction of stereochemically-dense carbocycles, including those present in many classes of bioactive, complex natural products and small molecules. We would like to thank the ACS PRF for their generous support of this project.