57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150307-DNI1: Fluorophobic Effects in Homogeneous Catalysis
Mark R. Biscoe, PhD, City University of New York (City College)
As described in the PRF progress report submitted last year, the general direction of research in our laboratory has evolved since the submission date for the original PRF grant application. Initially, we proposed to explore the effects of fluorophobicity on transition metal-catalyzed reactions. The over-reaching goal of this proposed research was the development of a novel strategy to generate value added materials from resources derived from petroleum feedstocks. The goal of developing new, useful methods to rapidly generate value added materials remains the general goal of our research program. The major goal of our current direction is the development of general methods by which to employ configurationally-stable, optically-active nucleophiles in transition metal-catalyzed carbon-carbon bond-forming reactions. By establishing the stereogenic center prior to the formation of the final desired bond, the rapid preparation of diverse libraries of single-enantiomer molecules would be achievable.
We previously reported general Ni-catalyzed processes
for the cross-coupling of secondary alkylzinc and tertiary alkylmagnesium
nucleophiles with aryl electrophiles.1a-1c These methods largely
circumvented the b-hydride elimination/reinsertion sequences that had
limited previous Pd-catalyzed systems. In order to expand the versatility of
cross-coupling reactions employing secondary alkyl nucleophiles, we sought to extend the processes to the use of
isolable, configurationally stable, optically-active
organometallic nucleophiles. The development of such transformations is impeded
by the inverse relationship that exists between the nucleophilicity
and configurational stability of carbon-metal bonds
in main group organometallic nucleophiles.2 While increased covalency tends to coincide with enhanced configurational stability of the carbon-metal bond, it also
tends to coincide with reduced nucleophilicity. This trend, in addition to the inherent
bulk of a secondary nucleophile, results in the prohibitively slow transmetallation of a secondary nucleophile as the covalency of the carbon-metal bond increases. Because secondary alkylboron
and secondary alkyltin reagents are most configurationally-stable, alkylbo
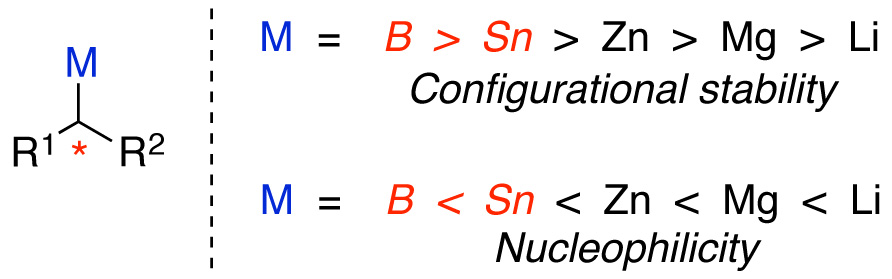 ron
and alkyltin are most conducive to development of a
general method for the use of optically-active nucleophiles in cross-coupling
reactions.
ron
and alkyltin are most conducive to development of a
general method for the use of optically-active nucleophiles in cross-coupling
reactions.

We
are excited to report that we have successfully developed a general Pd-catalyzed process for the cross-coupling of secondary
alkyl azastannatrane nucleophiles and aryl electrophiles.3 This reaction
displays no dependence on the electronic characteristics of either coupling
partner and occurs with minimal concurrent isomerization of the secondary alkyltin nucleophile.
Aryl chlorides, bromides, iodides, and triflates
are all viable electrophiles in this process. Additionally, optically-active
secondary alkyl azastannatranes undergo cross-coupling reactions with retention
of absolute configuration using this method. This process constitutes the first
general method to employ secondary alkyltin reagents
in cross-coupling reactions.
Overall, the combined generality of the transformation and stability of optically-active stannatranes
result in a process that should accommodate the broad use of optically-active
nucleophiles.
Having
established a viable method to employ optically-active secondary alkyltin
reagents in cross-coupling reactions, we are beginning to explore the
possibility of extending this process to the use of secondary alkylboron reagents. During these investigations, we have
developed a new, mild Pd-catalyzed method by which to generate primary
alkylboron compounds
from primary alkyl chlorides using
bis(pinacolato)diboron as a boron source.4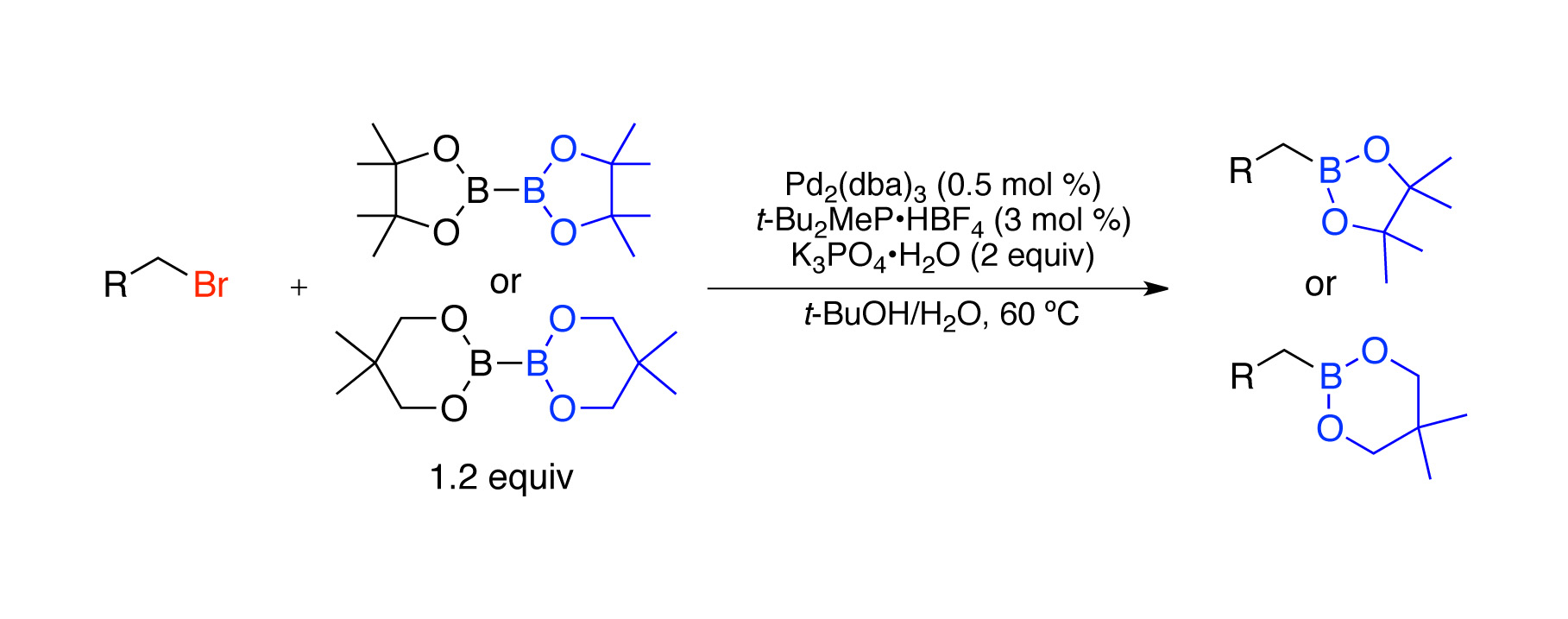
This
process accommodates the use of a wide range of functional groups on the alkyl
bromide substrate. Primary bromides
react with complete selectivity in the presence of a secondary bromide. The generality of this approach is additionally
demonstrated by its extension to the use of alkyl iodides and alkyl tosylates,
as well as borylation reactions employing bis(neopentyl glycolato)diboron as
the boron source.
The secured funding from the ACS Petroleum Research Fund has facilitated the development of the groundwork for the direct transition metal-catalyzed cross-coupling reaction of optically-active main group nucleophiles and aryl electrophiles. We have demonstrated that optically-active secondary alkyl azastannatrane reagents can undergo a Pd-catalyzed cross-coupling reaction with retention of absolute configuration. We are continuing to investigate the scope of this process, while also attempting to extend the method to alkylboron nucleophiles. We expect that methods that enable the rapid creation of optically active molecules will have immediate applications in medicinal and material sciences. This work will support future solicitations of funding from federal and private agencies, including a NSF CAREER proposal in the summer of 2013. Students participating in this research have gained valuable experience in the field of organometallic catalysis. Such transition metal-catalyzed methods are routinely employed in the fields of medicinal chemistry and drug discovery.
REFERENCES
1) (a) Joshi-Pangu, A.; Ganesh, M.; Biscoe, M. R. Org. Lett. 2011, 13, 1218. (b) Joshi-Pangu, A.; Wang, C.-Y.; Biscoe, M. R. J. Am Chem. Soc. 2011, 133, 8478. (c) Joshi-Pangu, A.; Biscoe, M. R. Synlett 2012, 23, 1103.
2) Boudier, A.; Bromm, L. O.; Lotz, M.; Knochel, P. Angew. Chem. Int. Ed. 2000, 39, 4414.
3) Li, L.; Wang, C.-Y.; Huang, R.; Biscoe, M. R. Submitted
4) Joshi-Pangu, A.; Ma, X.; Diane, M.; Iqbal, S.; Kribs, R. J.; Huang, R.; Wang, C.-Y.; Biscoe, M. R. J. Org. Chem. 2012, 77, 6629.










