57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR150115-UR1: Expanding the Scope of the Diels-Alder Reaction: Development of Cationic Dienophiles Stabilized by Cobalt-Complexed Alkynes
Kevin M. Shea, Smith College
As described in my original proposal, the goal of this project is to develop a new class of cationic Diels-Alder dienophiles. Our guiding principle is to exploit the cationic stabilizing ability of cobalt-complexed alkynes, and our hypothesis is that cationic dienophiles incorporating cobalt will react faster and more efficiently with a variety of dienes. We planned to examine the reactivity of several cationic dienophiles and then explore their reactivity in tandem Diels-Alder/Pauson-Khand reactions for the synthesis of complex polycycles.
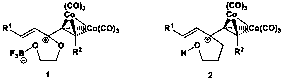
Our initial investigations focused on the development of two different classes of dienophiles: Gassman (1) and non-Gassman (2) types. Named for Paul Gassman, the pioneer in the study of cationic dienophiles, our Gassman-type dienophiles incorporate an oxygen atom that, along with the cobalt-complexed alkyne, stabilizes the reactive cation. In contrast, the non-Gassman-type dienophiles have a cation only stabilized by an adjacent cobalt-complexed alkyne. Like last year, we focused our research efforts this year exclusively on the Gassman-type dienophiles.
Scheme 1. Examples of a Gassman- and a non-Gassman-type dienophile
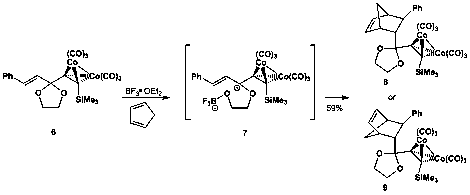
As reported previously, we have a robust synthesis of cobalt-containing dienophile precursor 6 as outlined in Scheme 2. Acetylide addition to cinnamaldehyde furnishes alcohol 3 (in 89% yield) which is subsequently oxidized in good yield to provide ketone 4. Ketalization with ethylene glycol yields ketal 5, and subsequent treatment with dicobalt octacarbonyl provides dienophile precursor 6.
Scheme 2. Synthesis of Gassman-type dienophile
To date, our best result is the reaction outlined in Scheme 3. Combination of 6 with BF3⋅ OEt2 and cyclopentadiene yields Diels-Alder product 8 or 9 in 59% yield. We are still investigating the stereochemical outcome of the reaction via 2D NMR and derivitization then recrystallization and X-ray analysis to determine which diastereomer is formed (vide infra). Reaction of 1,3-cyclohexadiene in a similar manner yields the corresponding Diels-Alder product, albeit in only 20% yield. Continuous investigation of other heterocyclic, carbocyclic, and acyclic dienes demonstrated that these are the only two reactions that proceed to yield significant quantities of product. Far from our initial hopes, our target dienophile reacts with a very limited scope. While examining the reactivity of 6 in the Diels-Alder reaction in comparison to 5, 4, and cobalt-complexed 4, we did demonstrate that 6 is the only compound that participates in the Diels-Alder reaction under our optimized conditions.
Scheme 3. Diels-Alder reaction of a Gassman-type dienophile with cyclopentadiene
Unfortunately, our efforts to unambiguously determine the stereochemistry of the Diels-Alder product are still underway. The presence of the cobalt in this compound complicated our ability to accurately analyze it by NMR. Thus, we have decomplexed this material to unmask the alkyne and remove the cobalt. This reaction proved more challenging than anticipated, but we have now developed two reliable procedures based on reactions with ethylenediamine and I2. This highlighted that we only synthesized one of the diastereomers. Now that our experimental hurdles are surmounted, we should be able to run key NMR experiments this semester. We are simultaneously working to synthesize derivatives of 8/9 that are crystalline. We are hopeful that liberation of the ketal and functionalization of the corresponding ketone as a hydrazone or related imine derivative will yield a solid that can be subsequently manipulated to yield a single crystal.
This PRF-funded research project has led to several important outcomes for my students and me. Most importantly, Sarah Rothstein (funded in the summer of 2011) completed a senior thesis on this research and presented her results at Smith and a local ACS Undergraduate Research Symposium. She is currently a research scientist using the skills she developed at Smith at Nalas Engineering, a start up company in Connecticut. Tessa Clark is continuing her research on this project as an independent study during her senior year. She will be looking for industry jobs in chemistry upon graduation. Gloria Ortiz, a junior, is also continuing her research during this academic year, and she is already planning to attend graduate school in chemistry beginning in 2014. Gloria is a very talented student who was recently named an ACS Scholar. I recently submitted materials for promotion to full professor which included the results from our PRF funded research. I am hopeful that evaluation by external reviewers and my Smith colleagues will result in promotion in the spring of 2013.