57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI549350-UNI5: Synthesis and Characterization of Molecular Monolayer Directed Nanoscale Catalysts
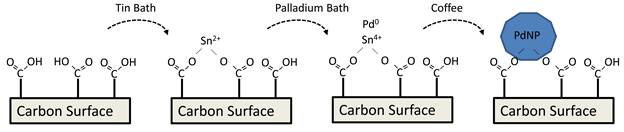
Kevin M. Metz, PhD, Albion College
The overarching goal of our research through this grant was to produce, study, and understand catalytically active metal nanoparticles on carbon substrates. Specifically, our research hoped to address: 1) the synthesis of uniform metal nanoparticles over large areas; 2) the synthesis of specialized (shaped and/or bimetallic) nanoparticles directly on substrates; and, 3) the catalytic activity of these particles.
In our first year of funding, our research efforts perused two similar avenues simultaneously. These were the creation and characterization of shaped palladium nanoparticles grown in situ on carbon substrates, and the creation and characterization of gold-palladium bimetallic nanoparticles grown in situ on carbon substrates. Both avenues used molecular monolayers to direct the synthesis of the nanoparticles on the surfaces. Due to thermal diffusion, we were unsuccessful in producing shaped palladium nanoparticles through the methods we attempted, which involved using the polyol synthesis at elevated temperature. While we were successful in producing gold nanoparticles directly onto molecular monolayer-modified carbon surfaces, we were not capable to produce a bimetallic system. This can be contributed to a lack of student effort.
In our second year, our focus shifted slightly due to difficulties in instrumental access. At that time, we had a long lag between producing samples and access to SEM for analysis. As a result, we needed to make sure that our systems of interest had a high probability of success before we spent time analyzing them. Thus, we moved away from molecular monolayer modified carbon substrates, and began using porous polycarbonate filtration membranes. This transition allowed for qualitative analysis of the synthesis of our nanoparticles, i.e., the membrane changed color when nanoparticles were fabricated. This color change provided the qualitative confirmation of nanoparticle formation needed to allow us to study of catalytic properties while we waited to access to microscopes. As a result of this transition to membrane substrates we were able to finalize our synthesis protocols and begin the characterization of catalytic behavior.
During our third year (first year of grant extenstion) two significant accomplishments were achieved. First, we developed a methodology to create metal nanoparticles using mild reductants at room temperature. Second, through collaboration with Prof. Paula Colavita of the School of Chemistry at Trinity College Dublin, Dublin, Ireland, we began studies on carbon microspheres as supports for our nanoparticles. The formed composites are very promising as catalyst systems.
Over the lifetime of this grant, 10 undergraduate research students have actively participated supported by ACS-PRF funds. Their efforts have resulted in presentations at the Wayne State University (Detroit, MI) Research Symposium in Chemistry, two presentations at the 239th National Meeting of the American Chemical Society (San Francisco, CA), three posters at the 241st National Meeting of the American Chemical Society (Anaheim, CA), and four poster presentation at the 243rd National Meeting of the American Chemical Society (San Diego, CA), and 4 poster presentation at the Midwestern Undergraduate Symposium on Chemical Research (MSU-R) held at Michigan State University (East Lansing, MI; 1 in 2011 that won best poster award, and 3 in 2012). Additionally, the work has produced three manuscripts, one that is currently under review and two that are being finalized for submission.
A brief summary of key
accomplishments to date is included below.
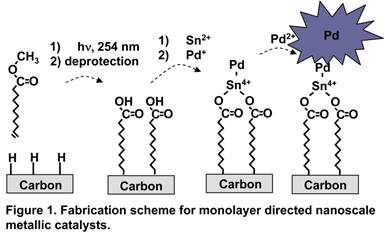
Our original proposed method of approach is outlined in figure 1. In this approach, carbon substrates are modified with molecular monolayers, which act as anchoring sites for tin ions through the formation of tin carboxylates. Once the tin carboxylate in bound to the surface, we proposed modifying chemistries developed in the 1950's and 1960's for plating metals on plastics to create our metal nanoparticles on the surface. While this approach did in fact work, it had low reproducibility. Furthermore, the variations observed in the results could never be correlated with experimental variations. One very important result did come from these studies, we discovered that many carbon substrates have sufficient carboxyl moieties in their native state for this scheme and do not require additional modification.
Figure 2. An illustration of the synthesis of palladium nanoparticles (PdNP) on carbon supports using coffee as a reductant.
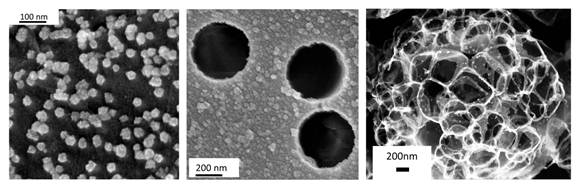
In the Spring of 2011 we solved our plating problems. We discovered an aqueous synthesis of palladium nanoparticles that used room temperature coffee and teas as a reductant. This approach could be transferred to our original proposed approach as shown in Figure 2. Using this approach, we were able to achieve highly reproducible results on a variety of substrates. Some of the substrates we have explored are shown in Figure 3. These include polymeric filtration membranes (Figure 3a), planer graphite substrates (Figure 3b), and carbon microspheres (Figure 3c).
While the planer graphite substrates do not display high catalytic activity, they do offer the ability to student fundamental aspects of our method. The polymeric filtration membranes allow for a flow-through reactor to be used in catalytic studies. The carbon microspheres are highly dispersible in a wide assortment of solvents, opening many possibilities for catalytic studies.
As mentioned above, we currently have a manuscript under review for the synthesis and catalytic application of palladium and silver nanoparticles on carbon microspheres. We are currently finishing a manuscript on the flow-through reactor design and hope to submit it before the new calendar year.
From here we would like to characterize the catalytic application of these systems in a few more reactions. Additionally, we would like to work on developing a method to make shaped nanoparticles at room temperature using natural reductants. This would be a significant improvement to current polyol techniques that require elevated temperatures for synthesis.