57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI149499-UNI1: Metathesis Reactions of Acyloxysulfones for Polyene Synthesis
Gregory W. O'Neil, PhD, Western Washington University
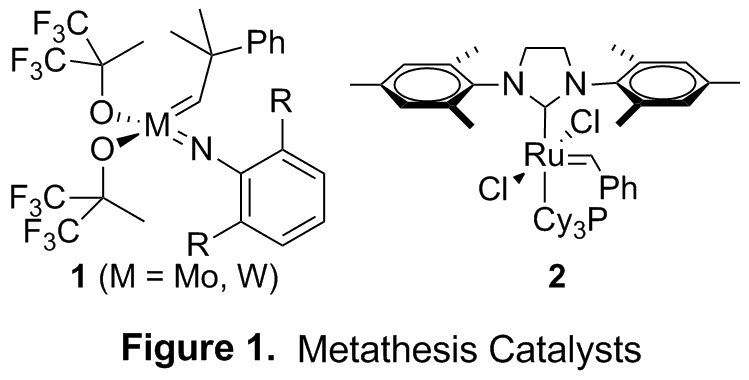
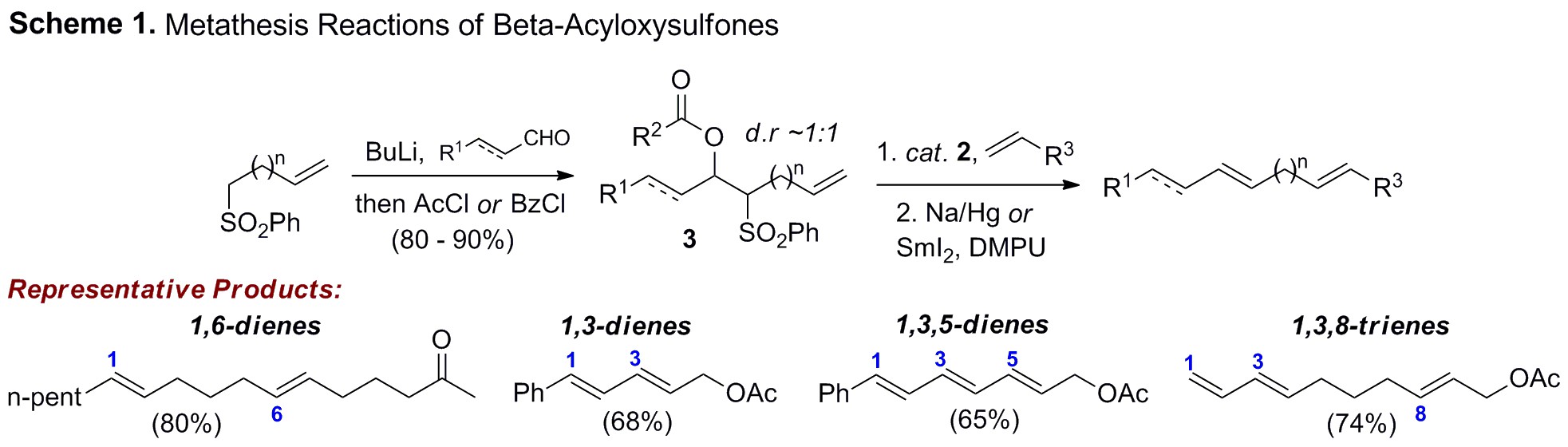
The impact of olefin metathesis on both organic and polymer synthesis cannot be understated. Despite the success of catalysts 1 and 2 in preparing a range of increasingly complex architectures, applications to polyene systems remain rare (Figure 1). This is largely the result of problems associated with chemoselectivity, or the nonselective engagement by the metathesis catalyst of one alkene in the presence of another.
Current efforts are focused on extending this strategy
toward increasingly challenging targets encompassing all general types of
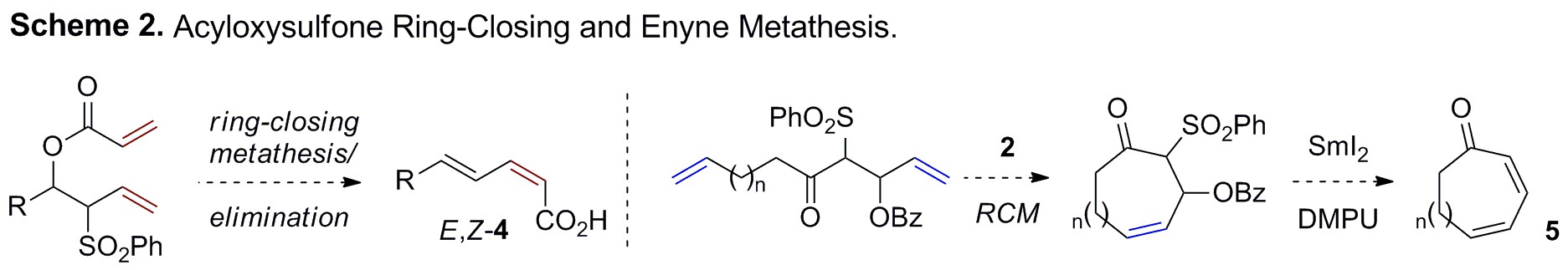
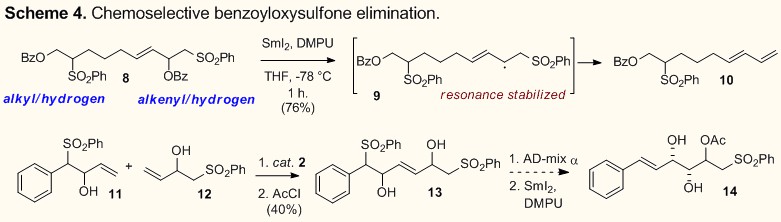
olefin metathesis reactivity. Examples include ring-closing acyloxysulfone
metathesis/elimination reactions to produce stereodefined
E,Z-dienoic
acids (4) and medium-ring dienyl-macrolactones (5)
(Scheme 2).
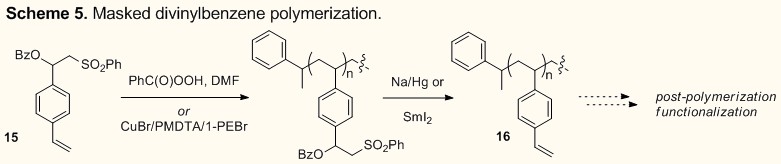
Recently we have also begun exploring
this general strategy for the synthesis of functionalized polymers (Scheme 5).
For instance masked divinylbenzene derivative 15 can be successfully polymerized
under both free radical and controlled radical conditions. Elimination then
gives 16 that is
poised for a variety of post-polymerization functionalizations.
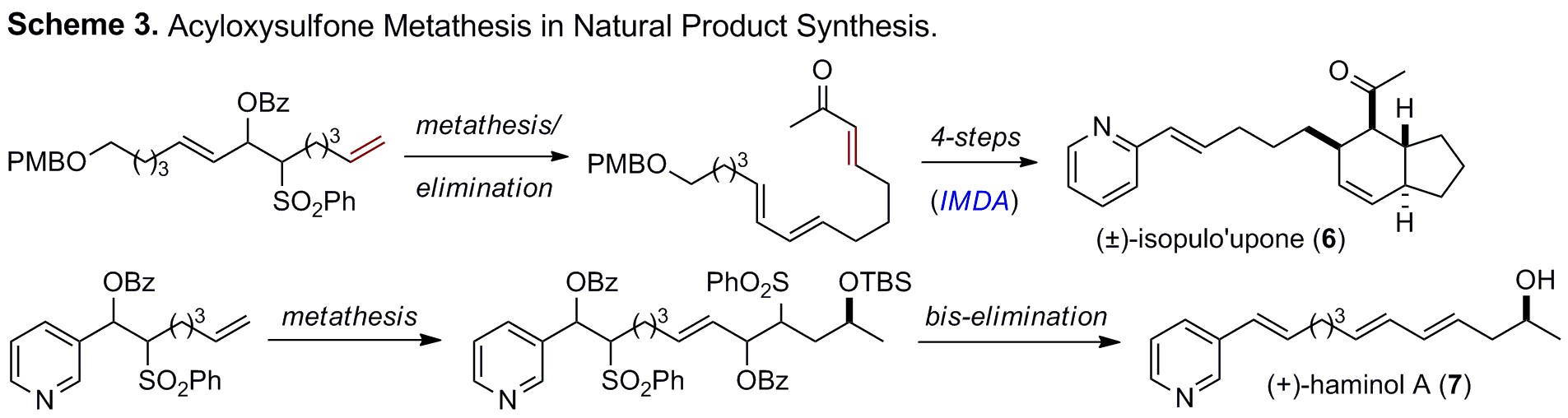
This work has provided the basis for
an ongoing research program aimed at the synthesis of biologically relevant and
environmentally compelling small molecules and materials utilizing acyloxysulfones as masked alkenes. Results were presented
by the PI and student participants at both regional and national meetings.
Student researchers supported by this grant during the past year have gone on
to pursue graduate degrees in chemistry at the University of North Carolina and
Boston University.