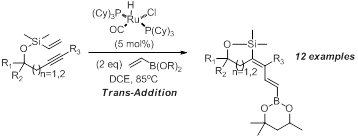57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150787-DNI1: Transformations Utilizing Ruthenium Hydrides for the Synthesis of Conjugated Systems
Daniel A. Clark, PhD, Syracuse University
The goal of this project was to develop a method for the synthesis of complex dienes. This work has the following objectives: (1) develop a method to incorporate acrylates into complex diene systems, (2) use ethylene to create highly substituted vinyl systems, and (3) explore the use of vinyl boronates to create dienes that contain both a vinyl silane and vinyl boronate moiety.
Findings: Tethered Vinylsilanes and Acrylates
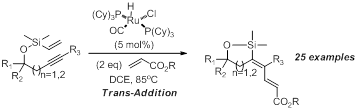
Initially we proposed the use ofalkynes that possessed a tethered vinylsilane could be coupled to olefins in the presence of ruthenium hydrides. Realization of our proposed goal would provide access to highly substituted dienes that are highly region- and stereoselective. As anticipated, we were able to create the desired dienes, however, we were able to ascertain that the dienes that were made did not possess the proposed stereochemistry. In fact, a rare trans-addition across the alkyne was observed. In fact, both simple and complex structures have been produces catalytically, using RuHCl(CO)(PCy3)2 as a catalyst. Specific examples of the accomplishments in this area include the use of both hindered and non-hindered acrylates as olefins. Both five and six-membered siloxane ring systems were created using our optimized conditions.
Our reaction is not limited to alkynes that are capped with an aryl group (R3). Alkyl groups such as methyl are well tolerated under the reaction conditions. Synthetic utility of our dienes was also demonstrated by vinyl silane to vinyl iodine exchange. The vinyl iodine was functionalized further using palladium catalysis. Of note, our method creates dienes which are difficult to make using currently available methods and accesses tetrasubstituted olefins that have four different carbon atoms.
Findings: Incorporation of Ethylene
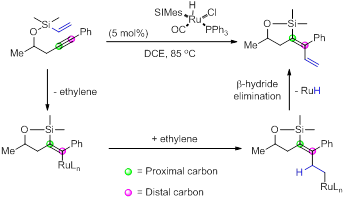
Our studies have expanded to include the use of ethylene as an olefin in this reaction. With the catalyst RuHCl(CO)(SIMes)PPh3 we have demonstrated that vinyl substituted dienes can be formed without the addition of excess ethylene. This serendipitous discovery arouse from our studies using acrylates. We observed the presence of a 1:1 mixture of acrylate product and vinylation product when the catalyst was used in the acrylate coupling. We found that this reaction was highly effective when the acrylate was removed and methyl vinyl ketone (MVK) was used as a co-catalyst. This protocol allows for straightforward entry into these diene systems. The only observed by-product in this reaction was the cycloisomerized six-membered ring product. In general, our findings indicate a broad substrate scope although rings larger than 7 have not been investigated.
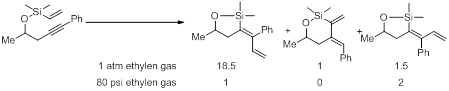
Of particular importance, we also observed the six-membered ring cycloisomerization product formation could be limited by the introduction of ethylene gas (1 atm). However, these conditions gave rise to isomeric products. In all cases the trans-addition was highly favored with cis-addition being the minor product and the cycloisomerization species was present only in trace amounts by nuclear magnetic resonance (NMR). The use of higher pressure of ethylene gas leads to a reversal in the product ratio. Using 80 psi of ethylene gas under identical conditions, the cis-addition was favored over the trans-addition product and the cycloisomerized product was not present by proton NMR.
Findings: Vinylboronates Containing Dienes
In an effort to broaden the scope of the dienes produced using the above methods we wanted to incorporate vinyl boronates into these systems. Our studies indicated that the hexylene glycol derived vinyl boronate performed well in this transformation. We observe only trans-addition across the alkyne which was anticipated from our prior work with acrylates.
Dienes synthesized with this methodology have been subjected to further functionalization using palladium catalyzed coupling to create higher substituted dienes and trienes.