57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND750813-ND7: Metallocene Catalyzed Polymerization Investigated by Hyperpolarized NMR
Christian Hilty, Dr. sc., Texas A&M University
Using DNP to Detect Intermediate Species in Polymerization Reaction
To facilitate progress on the development of the hyperpolarization technique applied to polymerization reactions, we performed initial experiments with a well characterized reaction, the polymerization of styrene. The dissolution dynamic nuclear polarization (DNP) experiment is a two-step process, where an aliquot of styrene with α,γ-bisdiphenylene-β-phenylallyl as polarizing agent was hyperpolarized at a temperature of 1.4 K, subsequently dissolved into dioxane, injected into the NMR spectrometer and mixed with an initiator solution consisting of sodium naphtalene in tetrahydrofuran (THF). Spectra of the reaction acquired after injection are shown in Figure 1a. As expected, strong signals of styrene are visible near 130 ppm. In addition, several peaks that do not stem from the monomer or the final polymer can be seen (highlighted in green in the sequence of spectra at the bottom). These peaks are attributed to the living anionic intermediate. In order to acquire the sequence of spectra shown, a fraction of the initially present spin polarization was detected at each time point. This use of hyperpolarization provides a unique way to assign peaks from the anionic intermediate species by correlation to the corresponding peak in the styrene monomer (i.e. the reactant). Specifically, after applying a selective inversion pulse to a monomer resonance at the beginning of the experiment, the transfer of the inverted signal to the anionic species was observed (Figure 1b). If the identity of the intermediate species is unknown, this correlation can be used for the assignment of its chemical shifts. The fact that the signals from the anionic intermediate in the on-going polymerization reaction could be identified and correlated to each of the peaks in the monomer is encouraging, as it demonstrates the potential of the real-time DNP-NMR method to yield mechanistic information on polymerization reactions. A manuscript describing these results is in preparation.
In view of the experimental protocol, it is important to note that the polymerization reaction occurred reliably after sample injection, despite the possibility for quenching by oxygen or water. In this case, the atmosphere with which the sample was in contact in the DNP and NMR instrument (helium and nitrogen liquid and gas is used) was sufficiently inert. A short exposure to air occurred during sample loading. In Figure 1, a peak at 143 ppm (as well as peaks at 36 ppm and 32 ppm) show the same chemical shifts as synthesized 1,4-diphenylbutane. These peaks can therefore likely be attributed to a fraction of polymer that has been quenched. Studying metallocene catalyzed polymerization, which is the goal of this project, poses the additional challenge of potentially increased air sensitivity. To this end, we have developed a new sample holder for DNP polarization that can be loaded and capped in a glove box and then transferred to the DNP instrument. Using the new holder, we are now planning to shift focus to these reactions.
Kinetic Modelling
An interesting feature of the hyperpolarized polymerization experiment has become apparent during the initial experiments. Due to a competition between spin-relaxation and reaction kinetics, actively growing polymer ends show the highest polarization levels. The active site of the reaction, which is usually the site of interest, therefore becomes selectively observable. We have developed a three-site kinetic model to describe this process, which considers the transfer of signal from the hyperpolarized monomer to the active site, and then to the interior of the polymer as a fresh monomer is added. The active site signal can show varying behavior, including monotonic decrease, or initial increase followed by monotonic decrease, based on the interplay between relaxation and kinetics. Since this is described by an equation that can only be solved numerically, reaction kinetics can most easily be determined from monomer signal. In the case of the styrene reaction, S(t)=Exp(-(k+r+λ)t), where k=kp*[I] is the pseudo-first order rate constant, kp the propagation rate constant, and [I] the initiator concentration. r is the monomer relaxation rate, and λ accounts for the depletion of signal due to readout of each scan with a radio-frequency pulse of small flip-angle. For a quantitative analysis of data sets measured for the styrene polymerization reaction (Figure 2), [I] was determined from mass spectra of the final product, and r from hyperpolarized experiments without addition of initiator. Fitting yielded propagation rate constants kp between 6.7 s-1 and 8.5 s-1 (in dioxane with 5% THF), which compares to literature values between 3.4 s-1 and 6.5 s-1 (in dioxane). Since literature values for rates in pure THF are substantially higher, the rates observed in our experiment in the mixture appear to be in good agreement with the literature. We conclude, therefore, that the method described here is capable of yielding quantitative results of polymerization kinetics.
Figures
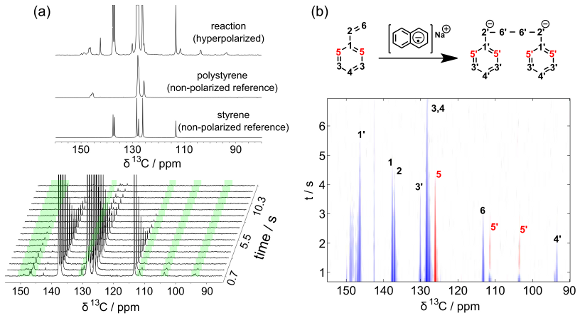
Figure 1: a) Hyperpolarized single scan spectrum of the progressing reaction (top). Time-series of a styrene polymerization reaction (bottom). Peaks from the carbanion intermediate are highlighted in green. b) Experiment with selective inversion of the ortho carbon in the styrene ring. Positive signals are indicated in blue, and negative signals in red.

Figure 2: Signal intensities of monomer peaks obtained from a polymerization of hyperpolarized polystyrene, including fit to kinetic model.










