57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI150466-UNI1: Novel Nucleophilic Trapping Reactions of Carbocations in Ionic Liquids
Elizabeth D. Kochly, PhD, Mills College
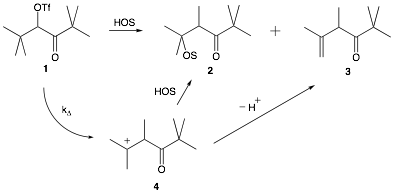
The main goals for our research are three-fold: 1) to expand our fundamental knowledge of carbocations, ionic liquids and the interactions between the two, 2) to synthesize and study novel organic compounds, and 3) to provide undergraduate women science students at Mills College an opportunity to perform directed research. Our current project is an investigation of the effect of ionic liquids on solute nucleophilicity. We are studying the solvolysis of pivaloyl triflate in binary solvent systems of ionic liquid and various organic alcohols. It is well known that pivaloyl triflate 1 solvolyzes via a kD process to yield rearranged products 2 and 3 (scheme 1). Upon formation of the rearranged carbocation, 4, the solvent acts either as a nucleophile or a base forming two potential products: substitution and elimination. We wished to determine whether the presence of ionic liquid would affect the product ratio.
Scheme 1. Solvolysis of pivaloyl triflate in a protic solvent.
In the previous annual report, work was reported on this solvolysis reaction carried out in solvent mixtures of bmimNTf2 with a variety of deuterated organic alcohols. It was found in all cases that as the percentage of ionic liquid co-solvent was increased, more elimination product was observed. This suggested that the alcohol co-solvent becomes more basic (rather than more nucleophilic) in the presence of ionic liquid. This work was recently submitted for publication to the Journal of Organic Chemistry.
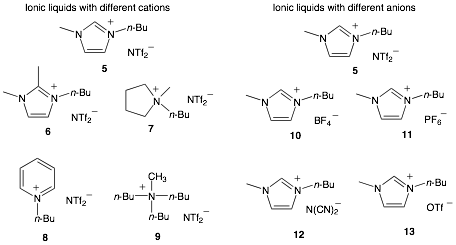
Over the past year, we have continued these studies to determine how the ionic liquid is affecting the reactivity of the co-solvent. Methanol was selected as the co-solvent and was combined with various ionic liquids. The ionic liquids 5-13, shown in figure 1, were selected so that the effects in changing the salt's cation could be observed independently of the effects of changing the anion.
Figure 1. The various ionic liquids used as co-solvents.
A series of reactions containing 5 % to 90 % ionic liquid co-solvent were run for each ionic liquid. It was determined that regardless of the ionic liquid chosen, elimination product increased with increasing ionic liquid concentration. The rate of increase, however, varied markedly. Some ionic liquids showed a small rate of increase while others showed a large rate of increase. This was explained by invoking Kamlet-Taft solvatochromic parameters of hydrogen-bond donating ability and hydrogen-bone accepting ability. If the positive ion of the ionic liquid is a good H-bond donor, the methanol co-solvent becomes less reactive and substitution product is favored. If the negative ion of the ionic liquid is a good H-bond acceptor, the methanol co-solvent becomes more reactive and elimination product is favored. These competing effects lead to the differences in product ratios seen with each ionic liquid.
Studies are ongoing to push the limits of these effects: an ionic liquid with an excellent H-bond donor cation and poor H-bond acceptor anion (to determine if substitution can ever be favored over elimination), and an ionic liquid with a poor H-bond donor cation and excellent H-bond acceptor anion (to determine if substitution product can be eliminated completely). We also intend to investigate more co-solvents to determine whether these results are unique to methanol or consistent among many protic co-solvents.
A primarily undergraduate institution, Mills College is a women's college with a substantial population of women of color. Funding from the ACS PRF has given some of these women valuable opportunities to participate in scientific research. Over the course of the last two years, five students have had the opportunity to work on these projects. Three of these students have now graduated. One has a successful job in industry. A second is now in a graduate program in chemistry. The third has completed a Masters of Public Health program at UCSF and is now atending medical school. The two remaining students are now students completing their degrees: a BS in Chemistry and a BS in biochemistry. All students have benefited greatly from working on these projects. This practical, hands-on experience has given these students valuable skills that have helped to prepare them for their future studies.
The principal investigator has also greatly benefitted from ACS PRF support. As a pre-tenure faculty member, this funding has allowed her to get her research up and running, and to submit a paper for publication in a peer-reviewed journal. She is currently writing up the results the studies described above for a second publication.