57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI350352-UNI3: Toward the Development of Iron-Based Olefin Metathesis Catalysts
Yann Schrodi, PhD, California State University (Northridge)
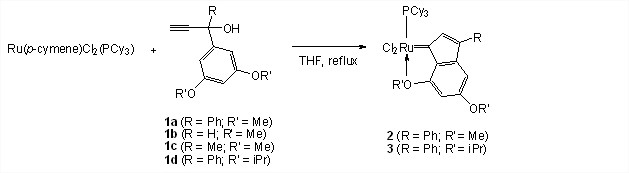
In recent years, our group has developed a straightforward and atom economic method for the preparation of a new olefin metathesis active ruthenium phenylindenylidene complex (2) by reacting RuCl2(p-cymene)(PCy3) with organic precursor 1a. In our previous progress report, we described exploring organic precursors 1b, 1c and 1d in similar reactions (Scheme 1). We published on the results related to 1b and 1c and on the use of different ruthenium starting materials [i.e., RuCl2(PPh3)3 and RuCl2(p-cymene)(L), where L is di-t-butylmethylphosphine, dicyclohexylphenylphosphine, triisobutylphosphine, triisopropylphosphine, or tri-n-propyl-phosphine] during this reporting period (Jimenez et al. Molecules 2012, 17, 5675-5689).
Scheme 1. One-step preparation of olefin metathesis active ruthenium indenylidene complexes.
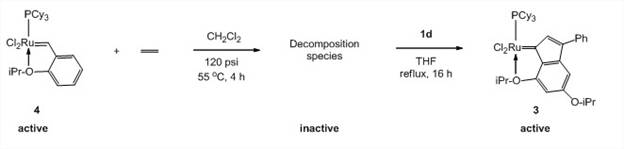
Organic precursor 1d can also be used to reactivate a decomposed 1st generation Hoveyda catalyst 4 (Scheme 2). This represents the first regeneration of a ruthenium-based olefin metathesis catalyst. A manuscript describing these results is being prepared and will be submitted in 2012.
Scheme 2. Regeneration of 1st generation Hoveyda catalyst (4) using organic precursor 1d.
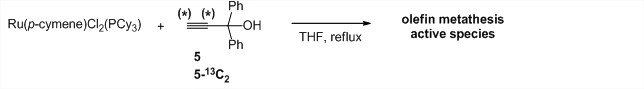
Interestingly, RuCl2(p-cymene)(PCy3) was found to react with commercially available 1,1-diphenyl-2-propyn-1-ol (5) in refluxing tetrahydrofuran to yield olefin metathesis active species, albeit less active than complexes 2 and 3 (Scheme 3). This is an attractive result, because it shows that an in-situ olefin metathesis catalyst can be prepared in one-step from commercial materials only. The nature of the active species remains poorly understood and further elucidation is being attempted by NMR spectroscopy using 5-13C2—an isotopologue of 5 labeled with 13C atoms in both acetylenic positions. The synthesis of 5-13C2 required the preparation of doubly-labeled 13C-acetylene from labeled barium carbonate, because 13C-acetylene is no longer available from Cambridge isotopes laboratories due to DOT regulations. Studies of the reaction between RuCl2(p-cymene)(PCy3) and 5-13C2 are ongoing.
Scheme 3. One-step generation of olefin metathesis active species from commercial materials.
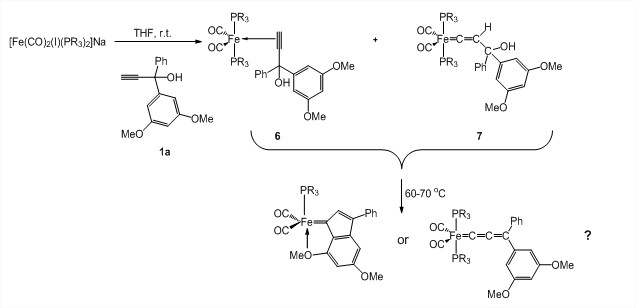
Attempts to prepare iron alkylidene complexes focused on the following two avenues: the treatment of iron dihalide materials LnFeX2 with di-Grignard reagents and the reaction of [Fe(CO)2(I)(PR3)2]Na with organic precursor 1a. The preparation of the previously reported di-Grignard reagents BrMg(CH2)3MgBr and BrMgCH2CMe2CH2MgBr proved very challenging. After considerable efforts and multiple attempts, we were never able to conclusively prepare BrMg(CH2)3MgBr and we generated BrMgCH2CMe2CH2MgBr in single-digit yields (averaging 8%). Considering, these difficulties and the fact that reactions of LnFeX2 and Grignard reagents do not yield clean products, we concentrated on the reaction between [Fe(CO)2(I)(PR3)2]Na and 1a. [Fe(CO)2(I)(PR3)2]Na (where R = OMe) was prepared from Fe(CO)5 in three steps according to literature procedures. [Fe(CO)2(I)(PR3)2]Na reacts readily with 1a in THF at room temperature to give putative π-complex 6 and vinylidene complex 7 as the main products after 10 minutes, according to NMR spectroscopy (Scheme 4). The reaction proceeds with conversion of 6 into 7. Upon heating the reaction mixture at 60-70 °C overnight, a new species is produced. Further characterization of species 6 and 7 and this new product is needed. In particular, we plan to react [Fe(CO)2(I)(PR3)2]Na with doubly labeled 1a-13C2 and study the structure of the Fe=[C] fragment by 13C NMR spectroscopy.
Scheme 4. Reaction between [Fe(CO)2(I)(PR3)2]Na (where R = OMe) and organic precursor 1a.
Overall, four undergraduate (Daniel Tolentino, Riccia Trinanes, Shuai Wang, and Aaron Wong) and five graduate students (Jordan Carter, Wing-Sy DeRieux, Steven Ruark, Matthew Ryan, and Daniel Tabari) participated in this project. During this reporting period, Shuai Wang and Aaron Wong received their BS degrees in Chemistry and Biochemistry and Matthew Ryan and Daniel Tabari received their MS degrees in Biochemistry. Shuai Wang is pursuing a PhD degree in Chemistry at UC Davis and Aaron Wong obtained a position as a technician at Medtronic Inc. Diabetes. Daniel Tabari started an MD program at Virginia Common Wealth University's School of Medicine. Daniel Tolentino and Wing-Sy DeRieux are currently applying to Chemistry PhD programs. This grant has undeniably provided these students with research opportunities that would not have been available to them otherwise. These opportunities have considerably enriched their education and experience and have changed their career paths.