57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI450272-DNI4: Self-Assembly of Directional Porphyrin Arrays in Water via Cyclodextrin-Based Host-Guest Interactions
Janarthanan Jayawickramarajah, PhD, Tulane University
Narrative Report Text
The main goal of this research project is to develop multi-porphyrin arrays in water (a highly competitive, yet sustainable and biocompatible solvent) using b-cyclodextrin (b-CD)-derived host-guest interactions. Specifically, we had proposed to (1) develop directional porphyrin arrays, and (2) to probe the energy transfer properties of the arrays. We have made substantial progress on these sub-aims as outlined below.
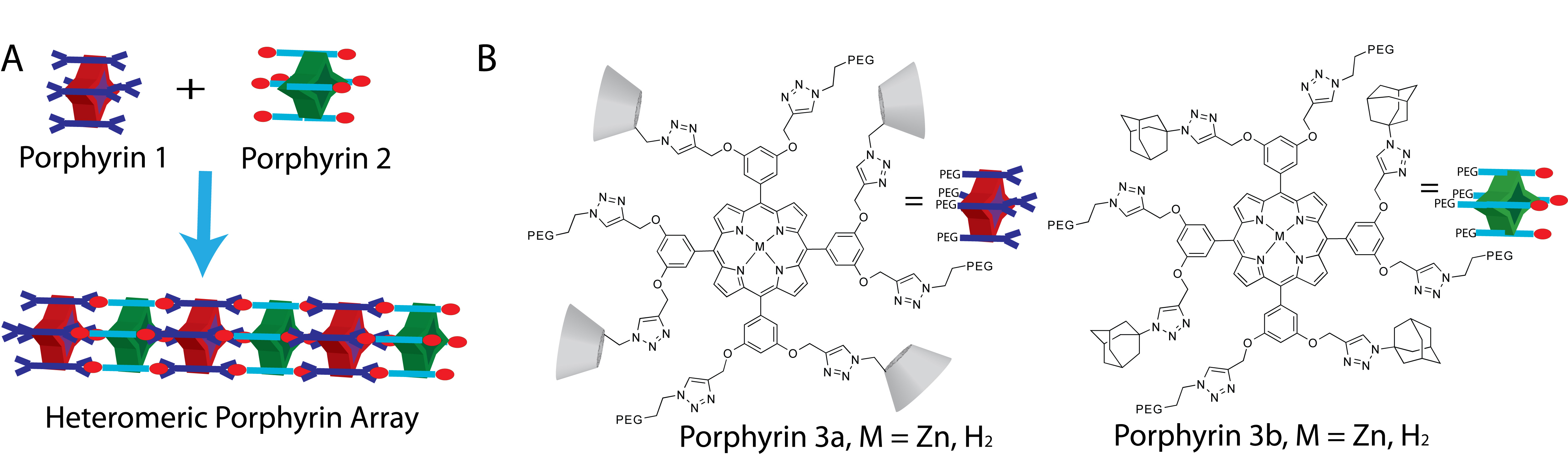
1. Progress on Trimer Self-Assembly. Based on our earlier (JACS 2010) publication that established the formation of linear porphyrin nanowires via multivalent b-CD/adamantane interactions (see heteromeric porphyrin array composed of porphyrins 1 and 2, in Figure 1A), one goal of ours was to prepare differentially functionalized porphyrin monomers that could be used for self-assembling well-defined smaller dimeric and trimeric systems. These model systems would then enable us to probe thermodynamic parameters of binding. Towards achieving this goal, we have completed the synthesis of four novel, water soluble, porphyrin derivatives functionalized with appropriate arms (either four adamantane arms or four cyclodextrin arms, whilst the other four arms are attached to water solubilizing PEG groups (see porphyrins 3a and 3b, in Figure 1B)). We are currently investigating the assembly of dimers (e.g., mixing 3a with 3b) and trimers (3b + 1 + 3b).
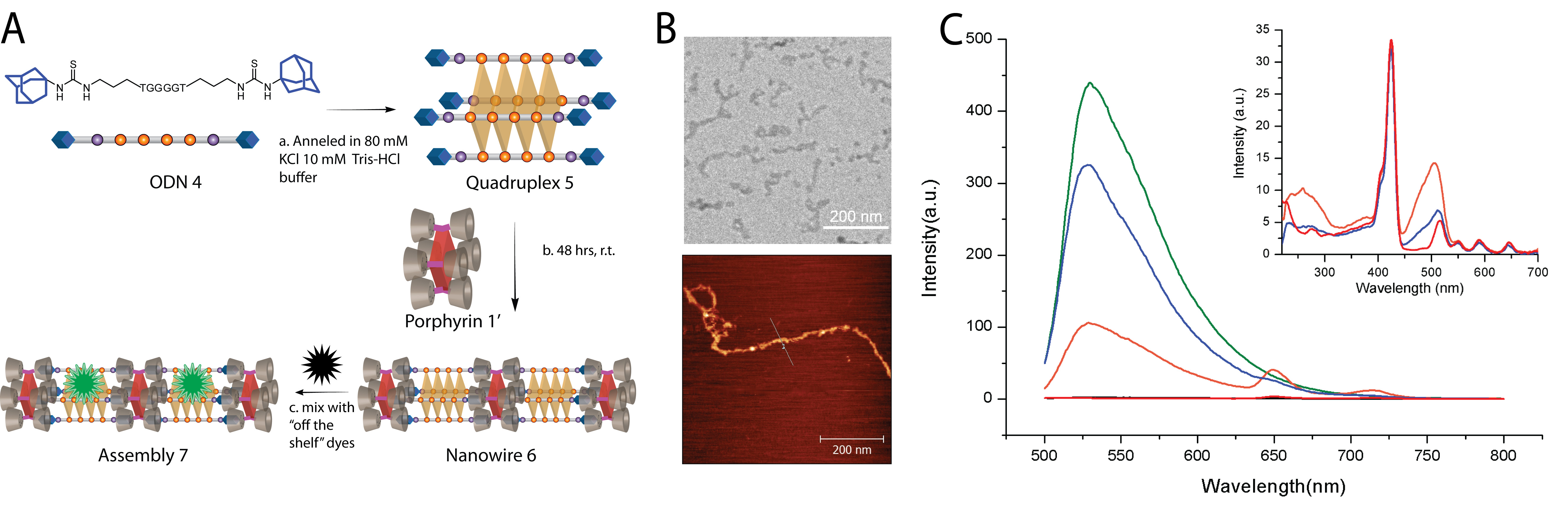
2. Progress on Preparing Multi-Porphyrin Arrays and Probing Energy Transfer Capacity. As mentioned in the previous progress report, we have developed multi-porphyrin arrays that are assembled via β-cyclodextrin/adamantane host-guest interactions in conjunction with tetramolecular guanine quadruplex formation. We are still investigating how adamantane containing guanine quadruplexes of varying length can modulate the inter-porphyrin separation. A distinct advantage of using this DNA quadruplex derived assembly is that the DNA domain allows for secondary molecular recognition events leading to the rapid construction of multi-chromophore arrays with broad-spectrum light-absorption. Specifically, we have shown that a DNA-porphyrin nanowire, composed of porphyrin 1¢ (free-base version of porphyrin 1) and quadruplex 5, can be further functionalized non-covalently with DNA binding dyes (see Figure 2A). The DNA-porphyrin array 6 and their higher-order bundles have been characterized inter alia via Cryo-TEM and AFM studies (Figure 2B). Further, we have used the DNA binding dye SYBR green 1 (SG), as an ancillary chromophore that can be loaded on the nanowires. As shown in Figure 2C, SG displays no appreciable fluorescence when excited (at 480 nm) in the absence of quadruplex 5. In contrast, the fluorescence emission of SG is enhanced 200 fold upon addition of quadruplex 5 clearly suggesting binding of SG with the DNA quadruplex. Interestingly, when SG is added to assembly 6 this enhancement in emission is diminished significantly and the presence of porphyrin emission bands at 650 and 715 nm is observed. The intensity of these bands are 10 fold higher than when only assembly 6 is excited at 480 nm (since porphyrin 1¢ has rather low absorption at 480 nm). Taken together, these experiments indicate that assembly 6 can be functionalized with SG resulting in a multi-chromophore array 7 and that an energy transfer process is present from the SG donor to the porphyrin acceptor. Additionally, we verified that the energy transfer process was due to a host-guest interaction held assembly. For example, when 7 is mixed with a large excess of free β-CD (250 equiv. per each β-CD unit on porphyrin 1¢), the fluorescence profile of the resulting solution shows that the high intensity emission (at 530 nm) corresponding to SG/quadruplex 5 is substantially restored and the emission from the porphyrin peaks is diminished. Further, the excitation spectra of the SG complexed array 7 (Figure 2C, inset) clearly shows that porphyrin emission can be induced by broadband excitation (from ca. 250 - 650 nm). Such defined multi-chromophore arrays that possess broad-spectrum absorption has potential importance in solar energy harvesting applications and remains difficult to achieve by traditional covalent and self-assembly methods. We are planning to submit a manuscript detailing this work shortly.
3. Other Multi-porphyrin Assemblies in the Nanoscale. We have developed a facile method to construct water-soluble porphyrinic nanoparticles, wherein the individual porphyrin chromophores do not undergo detrimental pi-pi interactions. Specifically, we have shown that incubation of a tetra-b-CD containing porphyrin (wherein two of the b-CD arms of the porphyrin encapsulate its core) with a,w diamino-polypropylene glycol (Mn = 2000 Da), followed by reaction of the accessible amine groups with fluorodinitrobenzene, can lead to the formation of well-defined nanoparticles. These fluorescent particles are being investigated by various techniques (including SEM and confocal microscopy).
4. Impact of PRF Funding on the PI's Research Program. As mentioned in the last progress report, ACS-PRF funding has been instrumental in the success of the PI's research program and career. The PRF grant has supported partial stipends for a number of researchers. One PhD student has graduated and is doing a post-doc at Northwestern University (with Prof. Fraser Stoddart). In addition, a second PhD student, Nan Zhang will be graduating soon. Further, this grant also laid the foundation for the PI to secure federal funding from the National Science Foundation.
Figure 1. (A) Self-assembly in water of octa-cyclodextrin functionalized porphyrin 1 in the presence of octa-adamantane porphyrin 2. (B) Tetradentate water-soluble porphyrins that have been synthesized, allowing us to potentially access smaller dimer and trimeric self-assemblies.
Figure 2. (A) Hierarchical construction of multi-chromophore containing assembly 7. (a) Self-assembly of bis-adamantane flanked ODN 4 into tetramolecular quadruplex 5. (b) Host-guest interactions derived assembly of quadruplex 5 and porphyrin 1' into multi-porphyrin containing array 6. (c) Incorporation of DNA-binding dye SYBR Green to form multi-chromophoric array 7. (B) Top: Cryo-TEM image of bundles of nanowire 6 in 160 mM KCl, 10 mM Tris-HCl, pH 7.5. Bottom: A 650 x 650 nm2 AFM image of single nanowire 6 on mica substrate. (C) Fluorescence emission profile and excitation profile (inset: observed at emission l = 715 nm) of array 6 (red), SG only (black), SG + quadruplex 5 (green), assembly 7 (orange), and assembly 7 + excess β-CD (blue). Note: All solutions were 4 mM in quadruplex DNA, porphyrin 1', and SG. Excitation l = 480 nm.