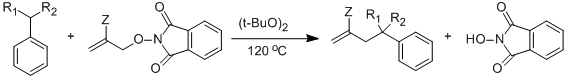57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: AC448587-AC4: 'Radically' Green Approaches to Hydrocarbon Functionalization Via Allyl Transfer
James M. Tanko, PhD, Virginia Polytechnic Institute and State University
1. Introduction
Hydrocarbon functionalization via an allyl transfer reaction (Scheme 1, X = Br) using various allyl bromide substrates, has been studied in our group. Replacement of Br• by phthalimido-N-oxyl (PINO•) was successful, and has helped make this chemistry environmentally benign. Reactions of allyl-phthalimido-N-oxyl (PINO) compounds for hydrocarbon functionalization have shown excellent results using high temperature initiators (di-tert-butylperoxide) and reactions are under investigation with respect to low temperature initiators (triethylborane, di-tert-bytylhyponitrite). Similarly, functionalization of ethers (tetrahydrofuran, 2-methyltetrahydrofuran, dioxane, diethyl ether etc.) has shown interesting results and is being studied further.
Scheme 1
The advantage of PINO• chemistry is the byproduct of the reaction, NHPI (N-hydroxyphthalimide), is easily recovered and separated as a while precipitate at the end of the reaction. Earlier, we reported that PINO chemistry works well with initiator DTBPO (di-tert-butylperoxide, a convenient source of t-butoxyl radical) leading to excellent yields. Also, we have reported the comparison between Br• and PINO• concluding that PINO• is more selective than Br• thus, PINO chemistry leads to high mass balance and better yields compared to Br• chemistry.
2. Experiments and Results
2.1 C-H bond functionalization of hydrocarbons: Overview
C-H functionalization of hydrocarbons has been previously studied and reported. A brief overview of the reported experiments and results are as follows
R1= H, CH3 R2=H, CH3 Z= COOEt, Ph Yield= Up to 91%, Time = 17-42 h
R1= H, CH3 R2=H, CH3 Z= COOEt, Ph Yield= Up to 65%, Low mass balance, Time =12-96 h
Scheme 2
The kinetic chain lengths of the both set of reactions were measured and it was concluded that former is a chain reaction with good chain lengths whereas, later is not an effiecient chain reaction (chain lengths =1-2). This difference is probably due to the slow β-fragmentation step at low temperatures.
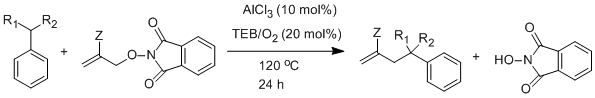
2.2 Lewis acid catalyzed Hydrocarbon functionalization
Primary objective of using triethylborane1 was to reduce the reaction temperature2, However this goal was not realized even at elevated temperature, reaction yields were low. A dramatic improvement in yield was seen when Lewis acid (AlCl3) was used along with triethylborane as initiator. These reactivity and mechanistic differences are under investigation using Laser flash photolysis.
R1= H, CH3; R2= H, CH3; Z = COOEt, Ph; Yield = Up to 89%; High mass balance
Time= 24 h
Scheme 3
2.3 Ether and thioether functionalization via allyl transfer reactions
The formation of new C-C bonds via the addition of carbon centered radicals is a useful method in organic synthesis. However, methods for the generation of α-ethereal carbon radicals are limited and difficult to carry out under mild reaction conditions. Since, ethers like THF, Me-THF, dioxane etc. has weak C-H bonds and resonance stabilization over neighboring heteroatom, our idea was to apply allyl transfer reaction strategy and carry out α-etheral C-H bond functionalization using di-tert butyl peroxide as initiator. We have successfully extended this research to ethers and thioethers with good yields and moderate selectivities.
Z= COOEt, Ph; Yield = 55-85%; Time =72-144 h
Scheme 4
Results are summarized in table 1
Ether | Z | Time (h) | Conc. of initiator Mol % | Yield % | Selectivity % |
THF | Ph/COOEt | 144/72 | 15 | 85/92 | - |
Me-THF | Ph/COOEt | 144/72 | 15 | 78/80 | 80:20 |
1,3 dioxane | Ph/COOET | 144/72 | 15 | 75/60 | 60:40 |
1,4 dioxane | Ph/COOEt | 144/72 | 15 | 70/72 | - |
Diethyl ether | Ph/COOEt | 144/72 | 15 | 60/64 | - |
Tetrahydrothiophene | Ph/COOEt | 144/72 | 15 | 75 | - |
Tert-butyl ethyl ether | Ph | 144 | 15 | 70 | - |
Tetrahydropyran | Ph | 144 | 15 | 80 | - |
Table 1
Both Me-THF and 1,3 dioxane have two reactive C-H bonds that lead to regioisomeric products. Selectivity was determined using GC and NMR spectroscopy. For Me-THF (80:20) and 1,3 dioxane (60:40). Major product was determined to be the one with the most reactive hydrogen on more substituted carbon.
2.4 Detail Study of 1,3 dioxane reactions to improve selectivity
Experiments and results for 1,3 dioxane functionalization are summarized in table 2.
Scheme 5
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
Table 2
2.5 Laser flash photolysis studies
Laser flash photolysis is currently being used to study the rate of the radical additions to allyl-PINO substrates. Various methods of generating benzyl and ethereal α-C radical have been tried. For benzyl radical addition to allyl-PINO substrates, a photolabile precursor3 of benzyl radical was synthesized and used for the generation of benzyl radical. These experiments are currently in process.
References:
1. (a) Miyabe, H.; Yamaoka, Y.; Takemoto, Y., Triethylborane-Induced Intermolecular Radical Addition to Ketimines. J. Org. Chem 2005, 70 (8), 3324-3327; (b) Nozaki, K.; Oshima, K.; Uchimoto, K., Et3B-induced radical addition of R3SnH to acetylenes and its application to cyclization reaction. J. Am. Chem. Soc. 2002, 109 (8), 2547-2549.
2. (a) Sorin, G.; Martinez Mallorquin, R.; Contie, Y.; Baralle, A.; Malacria, M.; Goddard, J.-P.; Fensterbank, L., Oxidation of Alkyl Trifluoroborates: An Opportunity for Tin-Free Radical Chemistry. Angewandte Chemie International Edition 2010, 49 (46), 8721-8723; (b) Zhang, Z.-C.; Chung, T. C. M., Reaction Mechanism of Borane/Oxygen Radical Initiators during the Polymerization of Fluoromonomers. Macromolecules 2006, 39 (16), 5187-5189.
3. Aveline, B. M.; Kochevar, I. E.; Redmond, R. W., Photochemistry of N-Hydroxypyridine-2-thione Derivatives: Involvement of the 2-Pyridylthiyl Radical in the Radical Chain Reaction Mechanism. J. Am. Chem. Soc. 1995, 117 (38), 9699-9708.