57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051423-DNI10: Coupling of Electrons, Photons, and Vibrations in Hydrocarbon Based Molecular Junctions
Jonathan A. Malen, PhD, Carnegie Mellon University
This project's goal is to experimentally probe the coupling of electrons to vibrations and photons in current-carrying hydrocarbon-based molecular junctions using measurements of Raman Spectra and photoconductance. Molecular junctions are formed with an organic molecule is bound between two inorganic electrodes. In order to understand how vibrations couple to electrons and photons, we have initially focused on understanding how vibrations within the molecule couple to phonons and electrons within the inorganic electrodes. To do so, we have formed self assembled monolayer (SAM) junctions and measured their thermal conductance using a thermoreflectance technique, in parallel to making first principles predictions of the thermal conductance with molecular dynamics.
The scientific impact of our
research is increase understanding of how phonons and electrons in the
inorganic contacts of a SAM junction couple and transmit through the discrete vibrational
levels of the SAM. In
semi-conductors and insulators, phonons are the dominant heat carriers, while
in metals electrons carry the heat. Prototypical molecular junctions and SAM junctions
are based on insulating molecules that hence carry heat by vibrations. Despite
a plethora of work on electronic properties in SAMs, there have been limited
developments on thermal transport in SAM junctions. Interfacial thermal
conductance (G) defines the
temperature difference DT
for a specified heat flux q" across
an interface,
![]() .
.
Experimentally a handful of measurements have considered the G of a SAM junction with asymmetric electrodes including GaAs-alkanethiol-Au 1 and Quartz-alkanesilane-Au 2. Computational work has instead focused on Au-alkanthiol-Au junctions and clear agreement with experiments has not been shown 3, 4.
In collaboration with Alan McGaughey (Professor of Mechanical Engineering at CMU), I am coadvising a PhD. student in MechE named Shubhaditya Majumdar, to study jointly make experimental measurements and computational predictions of SAM thermal conductance. Several other students, Keith Regner, Zonghui Su, and Wee-Liat Ong have made minor contributions to the experimental setup. In developing experiments to measure the thermal conductance of SAMs our focus has been to create junctions that could be readily simulated and had novelty relative to what has already been measured. We decided that Au-alkanethiol-Au junctions made the most sense since they can eventually be modeled using well known molecular dynamics potentials and their electronic properties have been extensively studied.
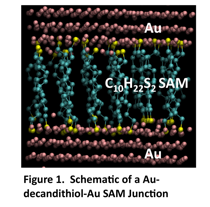
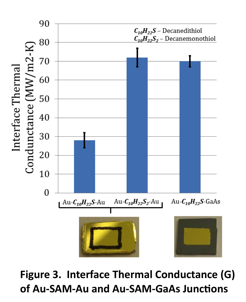
We have used the frequency domain thermoreflectance technique to measure Au-alkanethiol-Au SAM junctions formed using a unique transfer printing technique. Initially we templated Au films to create very flat surfaces to grow the SAMs on top of. To do so, we sputtered 500 nm of Au onto a Si wafer, then epoxied a tab wafer on top of the Au. After the epoxy hardened we pulled off the tab wafer, which picked up the Au layer, such that the exposed surface had sub-nm roughness as templated by the Si wafer. We formed decanedithiol and decanemonothiol SAMs on this layer from both ethanol and toluene solvents based on literature preps. These SAMs were then characterized by ellipsometry to confirm the presence of a single monolayer. To probe heat transport through a SAM junction it is necessary to apply a top contact. Electronic measurements have shown that forming a top contact by physical vapor deposition destroys the SAM. We have used the transfer printing process, pioneered by John Rogers lab and used for similar measruments by Losego et al. to transfer a 100nm Au top contact to the SAM. The final sample is (100nm Au top contact)-(decanethiol SAM)-(500nm Au bottom contact)-(Epoxy). A schematic of the Au-Alkanedithiol-Au SAM junction is shown in Figure 1. Photographs of the actual transfer prints are shown in Figure 3 under the plot, where we are showing Au-SAM-Au and Au-SAM-GaAs junctions for comparison.
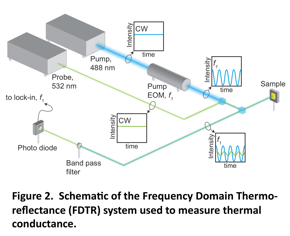
The SAM junction was measured using an optical technique called frequency domain thermoreflectance. The FDTR technique, shown in Figure 2, employs two continuous wave lasers to heat the sample and measure its thermal response to identify the unknown SAM thermal conductance. A 488 nm laser ("the pump") is intensity-modulated by an electro-optic modulator and periodically heats the gold surface while the 532 nm laser ("the probe") continuously monitors the resultant thermal response through the thermo-reflectance of the gold layer. A set of frequency-dependent phase data, related to the thermal properties of the sample are fit by a thermal conduction model to determine the unknown SAM thermal conductance 5.
Our results to date are shown in Figure 3 and indicate that the Au-SAM-Au junction has G of similar magnitude to asymmetric junctions measured before which have G = 25-60 MW/m2-K. Three preliminary conclusions are:
(1) G of symmetric SAM is strongly influenced by the head group. The decendithiol (C10H22S2), which makes covalent thiol bonds with both electrodes, has a much higher overall G than the decanemonothiol (C10H22S2), which makes a Van der Waals bond with the electrode on one side. This result agrees with Losego et al. 2
(2) G of an asymmetric GaAs-SAM-Au tested as a control is higher than found by prior literature 1, possibly because we have achieved higher areal contact between the SAM and top electrode. All of these measurements have been made at 300K. We intend to make measurements as a function of temperature next.
(3) G of the symmetric Au-SAM-Au is still much lower than molecular dynamics based predictions, which range from 250-400 MW/m2-K 3, 4.
1. Wang, R. Y.; Segalman, R. A.; Majumdar, A. Applied Physics Letters 2006, 89, (17), 173113.
2. Losego, M. D.; Grady, M. E.; Sottos, N. R.; Cahill, D. G.; Braun, P. V. Nature Materials 2012, 11, (6), 502-506.
3. Duda, J. C.; Saltonstall, C. B.; Norris, P. M.; Hopkins, P. E. Journal of Chemical Physics 2011, 134, (9), 094704.
4. Luo, T. F.; Lloyd, J. R. International Journal of Heat and Mass Transfer 2010, 53, (1-3), 1-11.
5. Cahill, D. G. Review of Scientific Instruments 2004, 75, (12), 5119-5122.