57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150491-DNI1: [3,3]-Rearrangements of O-Vinyl Oximes: Stereoselective Synthesis of 1,4-Dicarbonyl Compounds
Laura Anderson, PhD, University of Illinois (Chicago)
The specific aim of the proposed project was to develop the [3,3] rearrangement reactivity of O-vinyl oximes as a route to stereochemically defined 1,4-dicarbonyl compounds. The overall goal of the program is to explore and develop the chemistry of O-vinyl oximes as valuable precursors to privileged synthetic intermediates to streamline syntheses of pharmaceutically active molecules and desirable new materials. The specific aim of the project built on our preliminary studies that showed that a variety of O-propenyl oximes are easily accessible from O-allyl oximes via an iridium-catalyzed isomerization reaction and that O-propenyl oximes can be subsequently thermally converted to pyrroles. During the first year of the funding period, we achieved the following: 1) discovered that O-vinyl oximes undergo both [1,3] and [3,3] rearrangements and that this reactivity can be controlled by substituent effects and reaction conditions to provide regioselective access to either 2,3,4- or 2,3,5-trisubstituted pyrroles; 2) applied our discovery of the [1,3] rearrangement of O-vinyl oximes to stereoselectively prepare α-imino ketones and aldehydes; 3) studied the mechanism of the [1,3] rearrangement of O-vinyl oximes; and 4) developed a new copper-mediated coupling for the preparation of O-vinyl oximes, which provided a general, modular, and stereocontrolled route to our required starting materials. During the second year of the funding period, we have further used copper-mediated etherification to prepare N-enoxyphthalimides and O-vinyl hydroximates which we have exploited as novel intermediates for the facile preparation of α-oxygenated ketones and α-arylated ketones, respectively. In addition, we have observed that an analogous copper-mediated coupling procedure provides direct access to N-vinyl nitrones from fluorenone oxime and that these compounds undergo thermal rearrangements to form spiroisoxazolines.
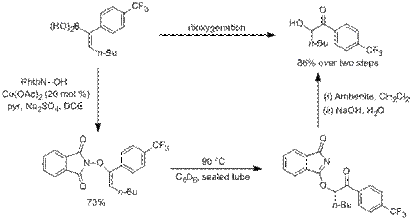
Scheme1: Dioxygenation of Vinyl Boronic Acids with N-Hydroxyphthalimide
Dioxygenation of vinyl boronic acids to give α-oxygenated ketones was achieved via the copper-mediated coupling of N-hydroxyphthalimide and vinyl boronic acids to give N-enoxyphthalimides that undergo subsequent [3,3] rearrangements to give the corresponding imidates which are easily hydrolyzed to give the desired products (Scheme 1). This method provides an alternative disconnection for the preparation of α-oxygenated ketones than currently employed transformations which rely on the oxygenation of enolates with electrophilic sources of oxygen. Specifically, it provides a means of directly converting an alkyne into an α-oxygenated ketone. Our initial publication of this work illustrates 22 examples of the preparation of N-enoxyphthalimides from N-hydroxyphthalimide, 8 examples of the conversion of N-enoxyphthalimides to α-hydroxyketones, and 8 examples of the conversion of N-enoxyphthalimides to α-benzoyloxyketones.1 Subsequent work on this portion of the project has identified a silyl ether derivative of N-hydroxyphthalimide that can achieve a similar vinyl boronic acid dioxygenation process with high diastereoselectivity.
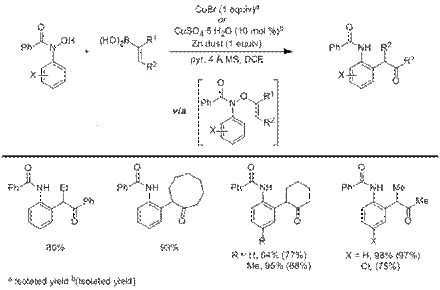
Scheme 2: Oxyarylation of Vinyl Boronic Acids with Hydroxamic Acids
Oxyarylation of vinyl boronic acids to give α-arylated ketones was similarly achieved via the copper-mediated coupling of N-aryl benzhydroxamic acids and vinyl boronic acids to give O-vinyl hydroximates which undergo a subsequent [3,3] rearrangement involving the N-aryl group (Scheme 2). This method provides a route to α-arylated ketones with o-amide substituents which currently cannot be accessed by palladium-coupling methods. Furthermore, the α-arylated products of this copper-mediated coupling and rearrangement of benzhydroxamic acids have analogous structures to an intermediate in the Fischer-Indole reaction which is generally challenging to detect due the subsequent facile cyclization to form indoles. It is noteworthy that, the mild conditions of the copper-coupling process provide reliable access to these valuable synthons. We currently have 12 examples of this transformation using conditions that require a stoichiometric amount of CuBr and 10 examples of an analogous transformation which only requires 10 mol % CuSO4 and 1 equiv of Zn dust. We are currently investigating diastereoselective and enantioselective versions of this transformation.
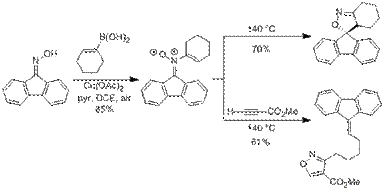
Scheme 3: Preparation of N-Vinyl Nitrones and Rearrangement to Spiroisoxaolines
N-Vinyl nitrones were prepared via the copper-mediated coupling of fluorenone oxime and vinyl boronic acids (Scheme 3). This general method for the preparation of N-vinyl nitrones is significant since the only previous report of an N-vinyl nitrone synthesis involved a multistep procedure with challenging intermediates. Facile access to these compounds has allowed us to investigate their reactivity. As shown in our manuscript which was recently accepted for publication, we have shown that these compounds undergo thermal structural rearrangements to form spiroisoxazolines as well as trapping and cycloreversion processes to form fluorene-tethered isoxazoles.2 We are currently investigating mechanistic trends to better understand when the copper-coupling conditions will favor O-vinyl oxime or N-vinyl nitrone formation. We are also pursuing the synthesis of N-vinyl nitrones of α,β-unsaturated oximes and their use in the preparation of highly substituted pyridines.
In summary, funding for this project has allowed us to explore the reactivity of O-vinyl oximes and show that these compounds are valuable new synthetic intermediates that can provide access to challenging structures via unique retrosynthetic disconnections. In year one, we discovered that enolizable O-vinyl oximes undergo [1,3] and [3,3] rearrangements to provide regioselective access to 2,3,4- and 2,3,5-trisubstituted pyrroles and that non-enolizable O-vinyl oximes undergo [1,3] rearrangements to give α-imino carbonyl compounds. During year two, we have further expanded the scope of our program to include the preparation and [3,3] rearrangement of N-enoxyphthalimides to give α-oxygenated ketones, the coupling of hydroxamic acids and boronic acids to give O-vinyl hydroximates that rearrange to give α-arylated ketones with o-amide functionalities, and the coupling of fluorenone oxime with vinyl boronic acids to give N-vinyl nitrones which undergo thermal structural rearrangements to give spiroisoxazolines. The diversity of the transformations of O-vinyl oximes, O-vinyl hydroxylamines, and N-vinyl nitrones that we have discovered over the past two years has laid a foundation for our research program and will continue to be pursued with other funding sources.
(1) Patil, A. S.; Mo, D.-L.; Wang, H.-Y.; Mueller, D. S.; Anderson, L. L. Angew. Chem. Int. Ed. 2012, 51, 7799.
(2) Mo, D.-L.; Wink, D. A.; Anderson, L. L. Org. Lett. 2012, in press.