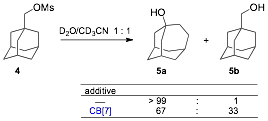57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND451053-ND4: Cucurbiturils in Organic Reactions: Encapsulation and Stabilization of Key Transient Species for Supramolecular Catalysis
Eric Masson, PhD, Ohio University
1. Introduction. There is currently enormous interest in the use of water as the solvent of choice in organic synthesis. In addition, the advent of supramolecular chemistry has given birth to new generations of tailor-made structures, which can direct the behavior of the various reaction partners in solution via host-guest interactions. Typical examples include calixarenes and cyclodextrins, which are now firmly settled in supramolecular territory. However, despite their outstanding recognition properties and their extreme binding affinities towards selected guests, the Cucurbit[n]uril (CB[n]) family of macrocycles has been rarely applied to supramolecular catalysis. Consequently, we proposed to identify and rationalize new CB[n]-catalyzed and CB[n]-promoted reactions in aqueous medium.
2. Impact. So far, this grant has allowed our post-doctoral associate to gather enough results for a full article that is currently in preparation (section 3.1), and to support the research expenses of two graduate and two undergraduate students, whose results will be incorporated into two additional publications in the coming months (sections 3.2 and 3.3). The PI has also exploited PRF funding to perform density functional calculations related to the post-doctoral associate's project, and to submit the outcomes to the journal Organic and Biomolecular Chemistry (section 3.1; manuscript currently under revision). PRF funding is clearly having a tremendously beneficial effect on our research activities, and is contributing to the preparation of new research proposals to public and private funding entities.
3. State of Research
3.1. CB[n] impact on torsional isomerization rates of substituted biphenyls. As proposed, we have encapsulated biphenyl units into the cavity of CB[8], and tested the impact of the macrocycle on the isomerization barrier along the aryl-aryl axis. We have opted for dimethyl(3-biphenyl)sulfonium derivatives 1, with substituents at the 2, 6 and 2'-positions. The sulfonium unit not only acts as a positive anchor that interacts with the carbonylated portal of CB[8], but also as a diastereotopic probe to assess the rate of torsional isomerization (see Figure); substituents were carefully chosen to guarantee torsional barriers in an energy interval measurable by dynamic nuclear magnetic resonance spectroscopy (DNMR) in aqueous medium (temperature between 0 and 85 oC, barriers between 13 and 18 kcal/mol). Remarkably, encapsulation of the biphenyl units dramatically enhanced the chemical shift differences between the two sets of methyl hydrogens at the sulfonium group (up to 0.36 ppm), and allowed the convenient determination of the isomerization barriers by line shape analysis at various temperatures (16.2 and 15.4 kcal/mol in the case of biphenyls 1a and 1b, respectively). Unfortunately, in the absence of the macrocycle, both methyl groups showed overlapped signals, both in 1H and 13C NMR spectroscopy, and the barriers could not be determined experimentally. Using a carefully benchmarked DFT method (see next paragraph), barriers of 15.4 and 14.4 kcal/mol were calculated for biphenyls 1a and 1b in the absence of CB[8]. We conclude that (1) CB[8] has a negligible effect on the rates of torsional isomerization, and (2) CB encapsulation allows the determination of otherwise inaccessible torsional barriers by exacerbating differences in electronic environments surrounding the probed hydrogen atoms.
When attempting to calculate the torsional barriers, we realized that density functionals and basis sets had never been screened with a large ensemble of biphenyl derivatives and a wide interval of barriers (approximately 5 – 45 kcal/mol). We ended up carrying out the benchmarking ourselves, and presented the results in a recent article submitted to Organic and Biomolecular Chemistry (manuscript under revision). We showed that the B3LYP and B97-D3 functionals allow the very accurate calculation of 46 torsional barriers, as long as triple-ζ or quadruple-ζ basis sets are used, all conformations are screened at these levels of theory and electronic energies are corrected with zero-point energies and entropic contributions. If the procedure described in the manuscript is applied, one can be 99% confident that 95% of future calculated barriers would be within 2.0 kcal/mol of experimental data. When barriers lower than 30 kcal/mol are considered, this tolerance shrinks to 1.3 kcal/mol, and rivals analytical accuracy. Since biphenyls are ubiquitous structures in organic chemistry, being able to access accurate estimates of their torsional barriers is of critical importance; this study allows just this, and is expected to be of significant interest to synthetic groups in academia and in the pharmaceutical industry.
3.2. CB[n]s as containers favoring cycloadditions. As proposed, we report for the first time a Diels-Alder cycloaddition promoted by CB[7] and CB[8] (rate enhancements of up to 2,500 and 10 times, respectively). Adducts 3 could be obtained quantitatively from furan derivatives 2 upon CB[7]-promoted intramolecular [4+2] cycloaddition at room temperature. In the absence of the macrocycles, conversions required much higher temperatures (80 oC or higher), and adducts 3b and 3c could only be formed in 25% and 15% yield, respectively, in equilibrium with furan derivatives 2b and 2c, with no convenient way to isolate them. To the best of our knowledge, CB[7] has become for the first time a necessary reaction partner to afford quantitative conversion of a reactant into a new product.
3.3. Stabilization of carbocations by CB[n]s, and impact on substitution reactions. As proposed, we have shown that CB[n]s can affect the kinetics of substitutions, when the reaction center sits close to the carbonylated portal of the macrocycles. We showed that the rate of hydrolysis of 1-adamantylmethyl mesylate (4), with the adamantyl unit encapsulated inside CB[7], is enhanced by up to 10 times. Much more surprisingly, CB[7] also affects the regiochemical outcome of the substitution: while 1-adamantylmethyl mesylate (4) undergoes a quantitative rearrangement to homoadamantyl alcohol (5a) upon hydrolysis in the absence of CB[7], a 2 : 1 mixture of the rearranged and non-rearranged alcohol 5b was obtained when the adamantyl unit was encapsulated inside the macrocycle. The underlying causes of such an unprecedented result are still under investigation.
4. Future directions. The second year of PRF support will be devoted to the impact of CB[n]s on (1) tentative intermolecular Diels-Alder cycloaddition, (2) competitive substitutions in the presence of two or more nucleophiles, and (3) the regioselectivity of classic rearrangements involving cyclopropylmethyl and terpinyl cations.