57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI649572-DNI6: Vibrational and Electronic Spectroscopy of Cationic Diamondoids
Nicolas C. Polfer, PhD, University of Florida
Instrumentation
One of the main aims of this proposal involved the construction of an instrument that allows deposition of cations and anions in an inert argon matrix. A schematic representation of this set-up is shown in Figure 1.
The basic premise is that cations and anions are deposited in layers to prevent their neutralization in charge exchange reactions.
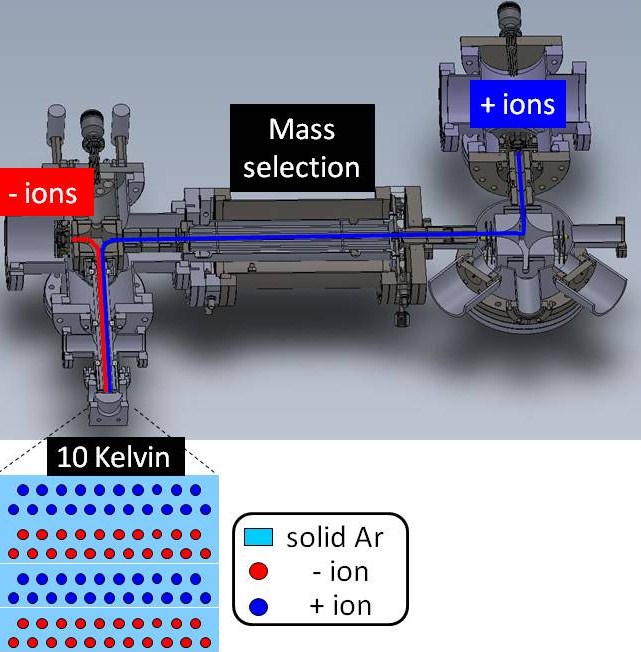
Figure 1. Schematic of custom-built mass spectrometer, showing ion sources for cations (blue) and anions (red), where both ion beams are guided to the cryostat window held at 10 K.
Formation of molecular hydrogen from hydrogenated polycyclic aromatic hydrocarbons
In some regions of the interstellar medium, the concentrations of molecular hydrogen, H2, are much more elevated than would be expected from existing models. The predominant view has long been that interstellar H2 is formed by H atom combination on small dust grains. Lately,
however, several new proposals have been made in which polycyclic aromatic hydrocarbons (PAHs) are thought to act as catalytic centers for the formation of H2.
The potential formation of H2 upon UV-vis activation of hydrogenated polycyclic aromatic hydrocarbons (PAHs), such as 9,10-dihydroanthracene (DHA), is investigated here.
A computation of the potential energy surface (PES) for DHA (Figure 1) shows that there is a low-energy pathway leading to direct formation of anthracene and molecular hydrogen.

Figure 2. Potential energy surface for H2 formation from 9,10-dihydroanthracene (at B3LYP/6-31G(d,p)).
Matrix isolation experiments
In order to simulate photolysis of PAHs on interstellar dust grains, DHA is embedded in a solid argon matrix at cryogenic temperatures (e.g. 12 K). The matrix is irradiated with UV-vis photons (at energies < 5.5 eV) from a mercury lamp. Infrared absorption spectra are recorded prior to and after photolysis, using an FT-IR absorption spectrometer. In addition, an absorption measurement on anthracene serves as a control experiment. Figure 2 displays IR spectra at different photolysis times, confirming that anthracene, and thus H2 are formed.
The rates of formation of molecular hydrogen in the interstellar medium can hence be estimated [1].

Figure 3. Infrared absorption spectra of neutral DHA trapped in solid Ar at 12 K (green), after irradiation with UV-visible photons from a 100 W Hg lamp for 4 hr (blue) and 21 hr (red). The infrared absorption spectrum of anthracene isolated in solid Ar (recorded in a separate experiment) is shown in black. Dashed lines indicate spectral bands unambiguously assigned to anthracene, while the open circles denote anthracene bands that overlap with DHA bands.
Scientific Meetings
The graduate student, Nathan Roehr, has presented updates on the custom-built instrument above at the International Symposium on Molecular Spectroscopy [2].
References
[1] Fu, Y.; Szczepanski, J.; Polfer, N.C.; Photon-induced formation of molecular hydrogen from a neutral polycyclic aromatic hydrocarbon: 9,10-dihydroanthracene. Ap. J. 2012, 744, 61-68.
[2] Roehr, N.; Szczepanski, J.; Polfer, N.C.; UV/Vis absorption experiments on mass selected cations by counter-ion introduction into an inert neon matrix. 67th International Symposium on Molecular Spectroscopy (Columbus, OH, June 18-22 2012).










