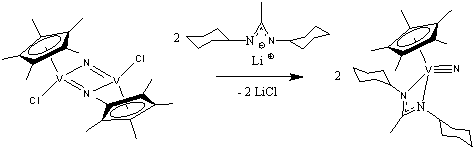57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR350700-UR3: The Synthesis and Characterization of Early Transition-Metal Complexes with Terminal Nitrido Ligands
Colin D. Abernethy, PhD, Sarah Lawrence College
The award of this PRF UR grant in the fall of 2010 coincided with my move to a new institution, Sarah Lawrence College in Bronxville NY. Since its founding in 1926, Sarah Lawrence has been a leader in pedagogical innovation; while the college has long been known for its strengths in the arts and humanities, my hire was part of a determined commitment on the part of the Board of Regents and senior administration to strengthen Sarah Lawrence's science programs and, in particular, to expand the participation of undergraduates in scientific research. As part of my start-up package, the college provided funds to equip a modern preparative laboratory for the synthesis and characterization of air-sensitive inorganic and organometallic compounds. At the present time my laboratory contains two vacuum/inert atmosphere Schlenk lines and an Innovative Technologies inert atmosphere glove box.
For the spring and much of the early summer of 2011, my students and I were occupied setting up our new laboratory. By July our preparations were complete and we were ready to begin the proposed research. The PRF grant was awarded to support our attempts to prepare new examples of early transition-metal complexes with terminal nitride ligands. Such species are, at the present time, quite rare, but are expected to exhibit very useful reactivity; for instance, as nitrogen-atom transfer reagents towards many important organic substrate molecules. Unfortunately, a majority of the few fully characterized examples of early transition-metal nitride complexes oligomerize via M-N-M bridges due to the nature of the highly polarized [M≡N] moiety, which combines a Lewis acidic metal center with the strongly basic nitride ligand. Our strategy is to employ combinations of very sterically demanding ancillary ligands to prevent the formation of M-N-M bridges and thus stabilize species with terminal nitrido ligands.
We began our investigations with an investigation of the metathesis chemistry of the easily-prepared vanadium(V) nitride dimer, [Cp*V(μ2-N)Cl]2 (1), with a variety of sterically demanding mono- and di-dentate anionic ligands. The first reaction we studied was that of 1 with the lithium salt of bis(cyclohexyl)methylamidinate. Our reasoning was that the replacement of the chloride ligands in 1 with sterically bulky didentate ligands would serve to break its V-N-V bridges and liberate a monomeric complex with a terminal V≡N moiety:
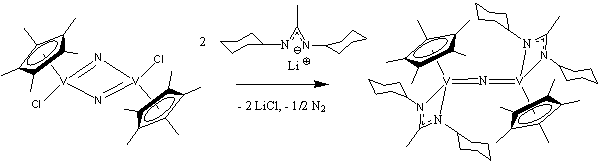
In the event, we discovered that, rather than forming a stable monomer with a terminal V≡N bond, this reaction consistently affords a mono(μ2-nitride) mixed-valent vanadium(III)/vanadium(IV) dinuclear species, [Cp*V(amidinate)]2(μ2-N) (2), in high yield with the concomitant evolution of dinitrogen gas:
The X-ray crystal structure of 2 shows a symmetric linear V=N=V bridge. In addition, the room temperature magnetic moment of 2, determined by the Evans NMR method, indicates that the species is paramagnetic with one unpaired electron per dimeric unit. These results are consistent with a description of 2 as a mixed-valence V(III)(μ2-N)V(IV) species with antiferromagnetic superexchange between the two vanadium centers.
In order to develop a rational mechanism for the formation of 2 and to discover how general this reductive elimination of N2 from [Cp*V(μ2-N)L]2 species may be, we have investigated the reactivity of 1 towards a variety of additional sterically demanding didentate nitrogen- and oxygen-donor ligands. We are currently waiting for the results of structural analyses of the products of these reactions.
To date, seven undergraduates have been involved in various aspects of this project. Preliminary reports of our findings were presented by the participating undergraduates as posters at both the 23rd Annual Harry C. Allen Jr. Chemistry Symposium at Clark University, Worcester MA (March 2012) and the Fall National Meeting of the American Chemical Society in Philadelphia, PA (August 2012).