57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR549516-UR5: Determination of Surface Mole Fractions in Mixed Binary AlkaneThiol Self-Assembled Monolayer Films on Gold by Thin-Layer Electrochemical Mass Spectrometry
Brian W. Gregory, PhD, Samford University
Introduction. This research has focused on examining the requirements necessary for using electrospray ionization ion trap mass spectrometry (ESI-IT-MS) as a quantitative tool for mixed alkanethiol self-assembled monolayers (SAMs). Our analyses have focused on negative-ion MS of the most stable oxidation product (alkylsulfonates, RSO3-) produced by reaction with H2O2. The development of ESI-IT-MS as a quantitative tool for SAMs should be applicable to SAMs more complex than those investigated here. With this work, my analytical capabilities have expanded into new areas (quantitative MS, thin-layer flow cells), while students have gained invaluable experience applying an important analytical tool (quantitative MS) to systems of fundamental interest in nanotechnology.
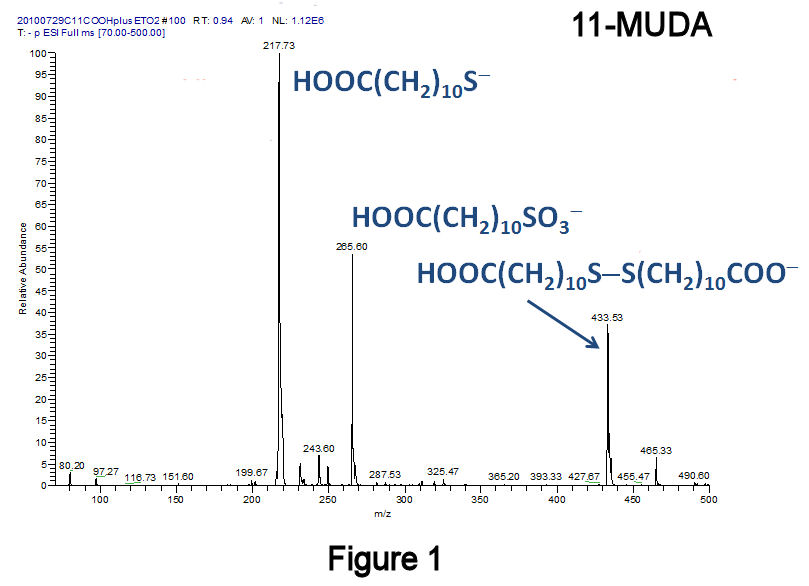
Elimination of Disulfides. This past year, studies of mercapto-alkanoic acid oxidation by H2O2 produced not only the desired alkylsulfonic acid, but also a disulfide-containing byproduct (Scheme 1: 11-mercaptoundecanoic acid, 11-MUDA). The former was observed by negative-ion ESI-IT-MS as the sulfonate, whereas the latter was observed as the carboxylate monoanion (Figure 1). The presence of disulfides was also indicated by the appearance of alkanethiolates, produced via heterolytic cleavage of the disulfide bond (Figure 1). These byproducts appeared quasi-stable to further oxidation, as their conversion to sulfonates was not accelerated by excess H2O2. Disulfides were not observed in our previous work with methyl-terminated alkanethiols since the former are electrically neutral. Thus, COOH-terminated alkanethiols have allowed us to probe the presence of various sulfur oxidation products by MS and to further optimize our methodology. Given our goal of producing only alkylsulfonates upon oxidation, the presence of disulfides is clearly undesirable.
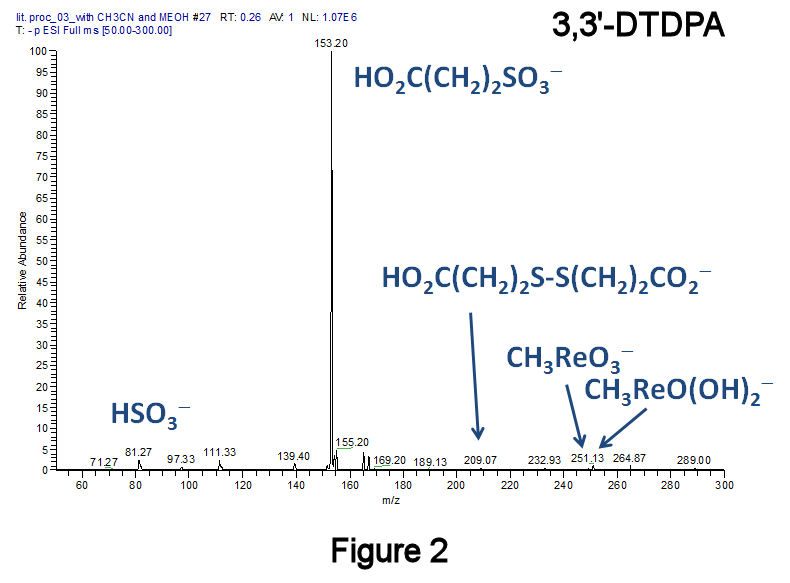
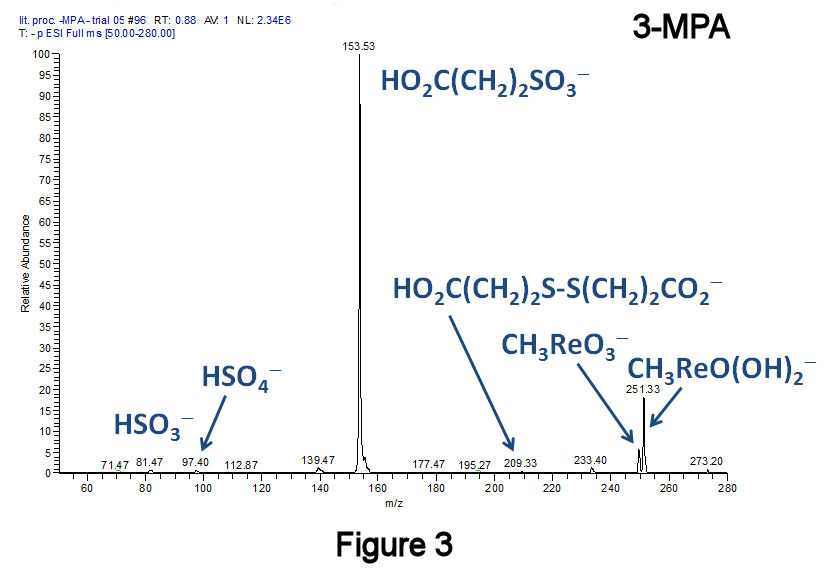
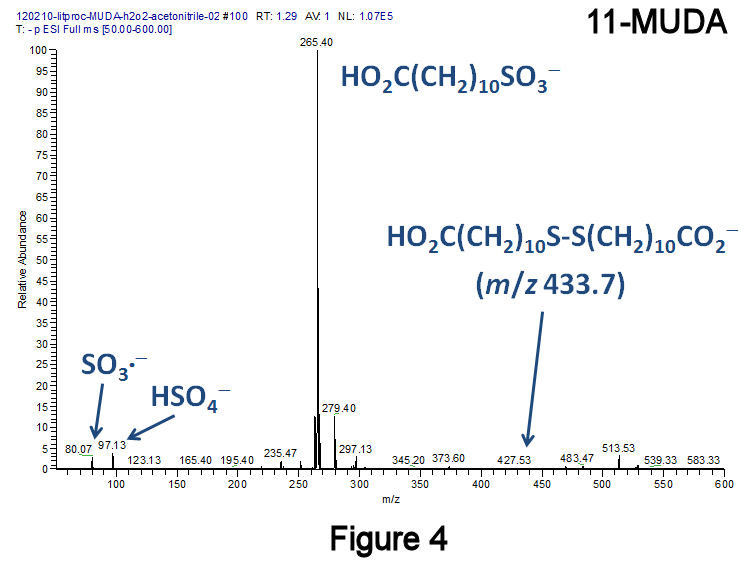
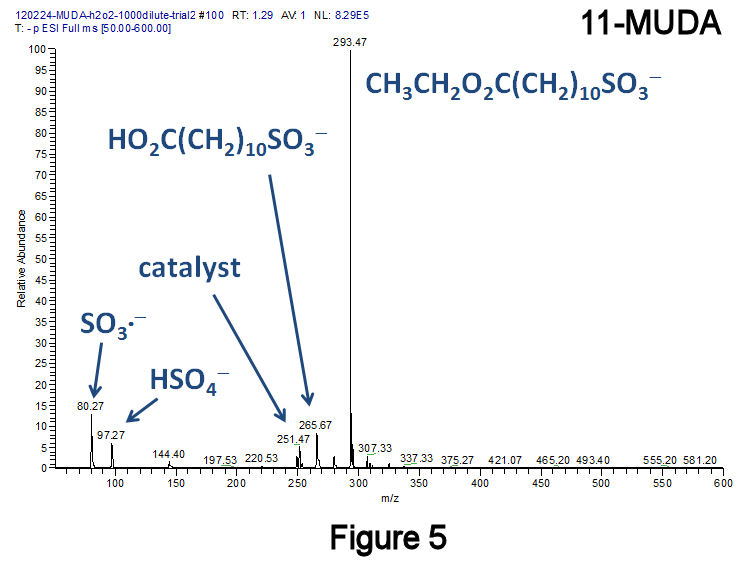
To eliminate disulfides, a recently published method was employed that converts diaryl- and dialkyldisulfides to the corresponding sulfonic acids at 25°C using H2O2 in combination with the catalyst methyltrioxorhenium(VII).[F.P. Ballistreri, et al., Tet. Lett. 2009, 50, 6231.] Using this method, a short-chain dialkyldisulfide (Figure 2: 3,3'-dithiodipropanoic acid, 3,3'-DTDPA), a short-chain alkanethiol (Figure 3: 3-mercaptopropanoic acid, 3-MPA), and a medium-chain length species (Figure 4: 11-MUDA) were investigated; in all three instances, almost no disulfide product was observed. Catalytic experiments performed with 11-MUDA in ethanol (where its solubility is greater) were also successful, although the majority of the sulfonate product became esterified (Figure 5). Using this catalyst, a 10-fold increase in the absolute MS signal intensity for 1-octanesulfonate has been observed (Figure 6), indicating that only 10% of the total alkanethiol may have been converted in our original approach. This catalyst has lead to significantly improved alkylsulfonate conversion efficiencies with very few changes to our original method.
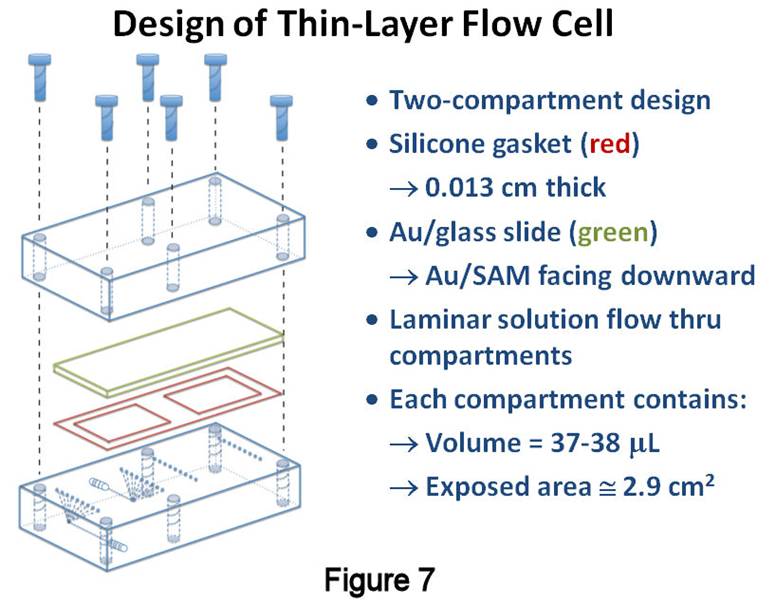
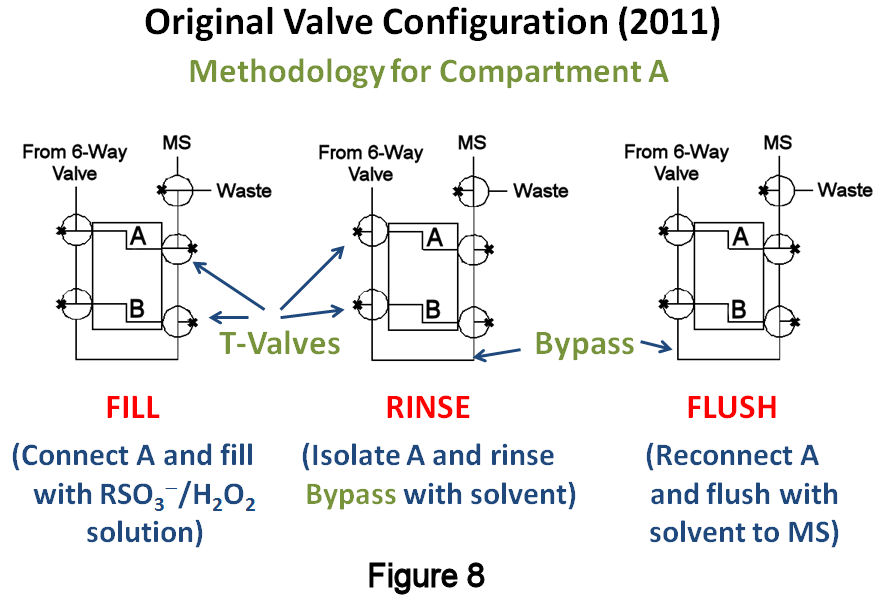
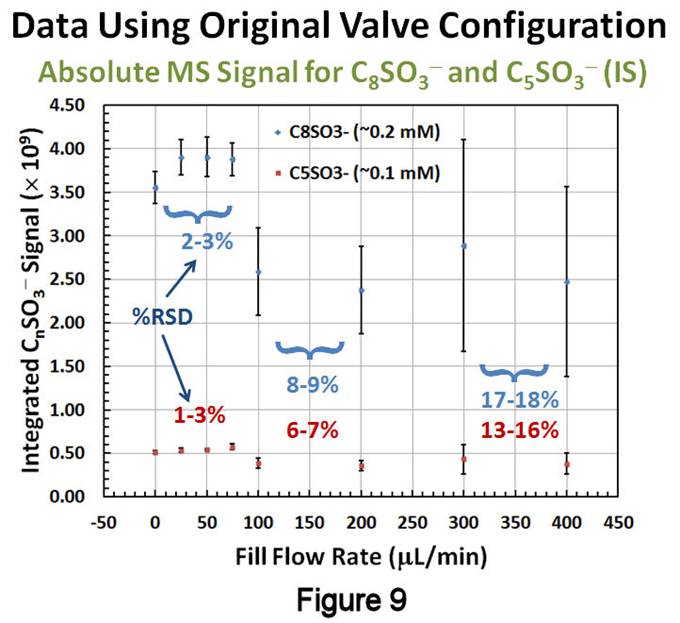
Thin-Layer Flow Cell Studies. Using this new catalytic method, studies resumed with the TLFC/valve system that was implemented the previous year (Figure 7). The TLFC volume is <40mL, whereas the total volume between the inlet and outlet T-valves was measured to be 193±7mL. Since this system should act like a reproducible sample loop, a closer examination of its performance characteristics was warranted. The methodology involves (Figure 8): (1) a "filling" step, where one of the compartments is filled with a solution containing H2O2, the alkylsulfonate of interest, and an internal standard (IS); (2) a "rinsing" step, whereby the compartment is isolated and the "bypass" around the TLFC is rinsed with blank solvent; and (3) a "flushing" step in which the compartment is reconnected in order to flush its contents to the MS for detection and quantification. The filling solution must contain an IS for reproducible quantification, as well as the alkylsulfonate of interest so that the measured signal ratio between the two is not below the limit of quantification after SAM removal. Given the small volume between the valves, it was presumed that passing >200mL of filling solution through the compartment might lead to transfer of desorbed SAM material past the outlet valve. Thus, initial experiments involved injecting the filling solution at a various flow rates and timing the filling step so that the solution would just fill the compartment. After rinsing the bypass, the contents were flushed to the MS at a fixed rate of 200mL/min. Figure 9 displays the results of these studies, showing that slower fill rates lead to improved absolute intensities, as well as smaller RSDs and 95% confidence intervals, for both 1-octanesulfonate (blue) and 1-pentanesulfonate (red, IS); similar results were obtained with plots involving the 1-octanesulfonate/IS signal ratio. Although the precision at slower filling rates was improved compared to last year's estimates (4.5%), it is still larger than desired (£ 1-2%RSD).
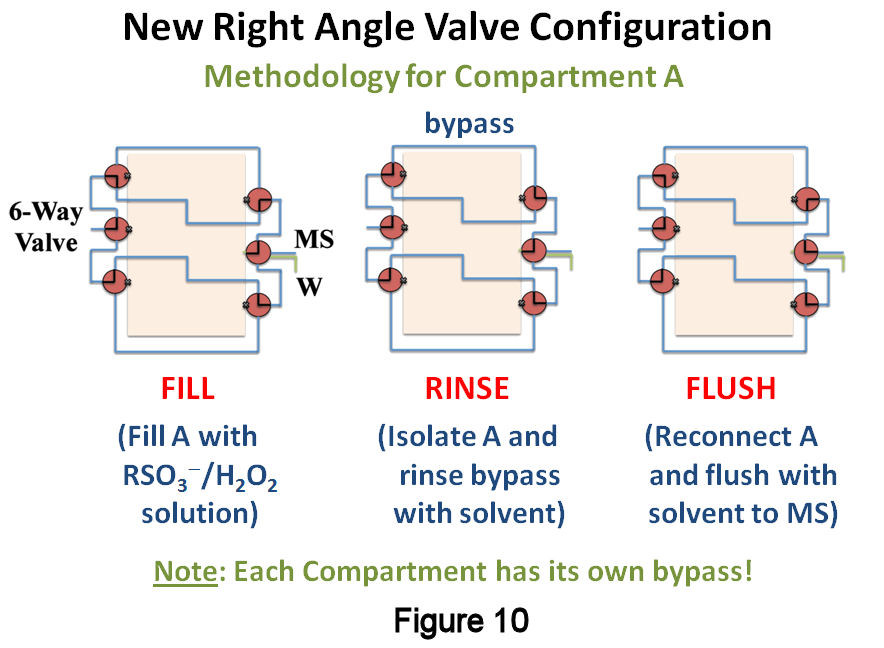
A potential source of irreproducibility was quickly identified as originating from our use of T-valves. During filling, these were operating as right-angle switching valves, resulting in a small dead volume contribution of 6.5mL to the total volume. This amounts to a 3% variability in the total volume, which can explain our smallest precisions at slower filling rates. The system was reconfigured with zero-dead volume, right-angle valves and two solvent bypasses, one for each compartment (Figure 10). Filling experiments were repeated as before, except that the MS signal of 1-octanesulfonate was monitored until it reached a constant value, signaling that the compartment had been completely filled. Figure 11 displays the results of these studies, where the data is plotted as a function of time after the filling solution was prepared and fill rates are shown above or below the points. From these data, several observations can be made: (1) the data show little dependence on fill rate; (2) at short times following preparation of the filling solution, the precision is excellent (<1%RSD) and the signal ratio is relatively large; and (3) as time progresses, a loss in signal and precision both occur. Since the filling solution is prepared from a concentrated stock solution produced via the catalytic approach discussed above, it is possible that slow changes in the extent of association between alkylsulfonates are occurring in the more dilute filing solution once it is prepared, and that these alter their ionization efficiencies over time. We are currently examining this effect and the means to eliminate it. Despite this, our reconfigured TLFC/valve system shows significant improvement in precision over the previous design.