57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND951641-ND9: Controlling the Partial Coalescence of Particle Stabilized Droplets
Ilona Kretzschmar, Dr., The City College of New York
David Harbottle, PhD, The City College of New York
Summary
We have studied the mechanism of coalescence for silica particle-laden aqueous droplets with planar hexadecane/water interfaces. The transition from partial droplet to complete coalescence depends on particle concentration, size, surface charge (electrolyte concentration), and wettability (surface functionality). This transition results from changes in coalescence kinetics, which is dependent on capillary wave propagation and horizontal neck collapse impeded by particle network jamming.
Introduction
In this past funding period, we studied the coalescence behavior of aqueous particle-laden droplets with particle-free, planar hexadecane/water interfaces to elucidate the effect of droplet rheology (particle-particle interactions) on coalescence behavior. As previously shown, capillary waves formed at film rupture, propagating along the droplet edge have been identified as driving mechanism for partial coalescence when considering particle-free, Newtonian fluids. Our study focuses on particle-laden droplets and the interplay of particle structuring and dampening of the capillary wave and its effect on suppression of partial coalescence.
To this effect, initial experiments with 250nm SiO2 particles considered effects of particle concentration and surface charge on coalescence behavior and were followed by experiments in which particle size and surface functionality were varied.
Experimental Details
All experiments described use silica particles (AngstromSphere®, diameters: 250, 500, and 1000nm). Initial particle batches were used as delivered, while later batches required treatment with 46% nitric acid overnight. Milli-Q grade water with a conductivity of ~0.05mS/cm is used as aqueous phase, while hexadecane (analytical grade) is used without further purification as immiscible light oil phase. 10-4M KNO3 is used as base electrolyte in all particle suspensions unless noted otherwise.
Particle wettability is varied through a surface silanation procedure with dimethyldichlorosilane in methanol.
Coalescence experiments are performed in a plexiglass cell scratch marked around the inner circumference at a height of 11mm to aid in pinning the oil/water interface (Fig1). Particle-laden droplets (3.9±0.2mm) are released from a plunger syringe with a 21 gauge needle ~3mm away from the interface. A Photron high-speed camera is used to record coalescence events.
Results and Discussion
We have studied the effect of particle concentration (0-30wt%), surface charge (10-4M vs. 1M), size (250, 500, and 1000nm), and wettability (hydrophilic vs. hydrophobic) on the transition from partial to full coalescence. Coalescence videos are analyzed using ImageJ and MATLAB® to track drop size reduction ratio defined as droplet diameter over initial droplet diameter (d/di). In the case of partial coalescence, d/di~0.3, while d/di=0 in the case of complete coalescence.
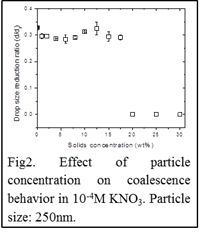
Fig2 shows the effect of particle concentration on the coalescence behavior of 250nm particle-laden droplets with a planar hexadecane/water interface. In a particle-free system, aqueous droplets always undergo partial coalescence with the interface as indicated by the black square. Partial coalescence is also observed when the solid fraction is in the 0-17.5wt% range. At higher particle concentrations the behavior changes from partial to complete coalescence. Bulk viscosity measurements at 0.2Pa show a substantial viscosity increase from ~0.005 (17.5wt%) to 0.0275Pa.s (20wt%) in direct correlation with the observed change in coalescence behavior.
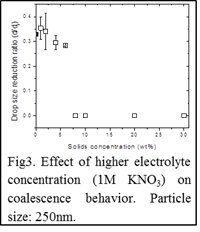
Increasing electrolyte concentration from 10-4 to 1M decreases the particle concentration (20 vs. 8wt%) needed to transition from partial to complete coalescence as shown in Fig3. Careful analysis of maximum droplet height and time to reach that height reveals decreasing height and shorter times for higher particle concentrations, which correlate with an increased dampening of the propagating capillary wave. Analysis of fluid neck width during coalescence gives insight into retardation of the horizontal collapse, which leads to partial coalescence. At high electrolyte concentration, particle aggregates readily form and potentially cause gelation of the particle-laden droplet, which in turn allows for stress transmission and contribution of an elastic component within the network. As a result, energy required for network break-up and further compression (horizontal neck collapse) increases with increased particle-particle interaction.
Most recently, we have started to investigate the effect of particle size on coalescence. We find (Fig4) that at constant particle concentration, droplets containing larger particles will transition from partial to complete coalescence at increased concentrations. This behavior may result from differences in particle density per unit volume and/or more pronounced gelation properties of finer particles.
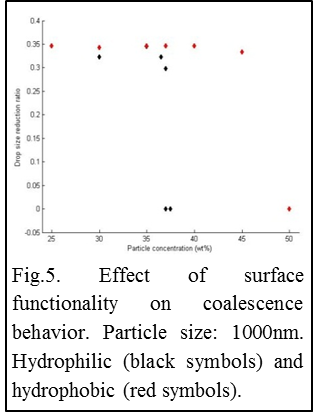
Preliminary data on the effect of particle wettability are shown for 1000nm silica particles (Fig5). From Fig5 it appears that hydrophobization of particle surface leads to retardation of partial coalescence to much higher weight fractions (37 vs. 47wt%). The measured difference may result from aggregation of hydrophobic silica when dispersed in a hydrophilic solvent, or from the interaction of particles at the droplet liquid-liquid interface.
Future Work:
To date the research program has generated interesting and thought provoking results that considerably contribute to the science of particle-laden and particle-stabilized (preliminary study) droplets at a planar-fluid interface.
Particle-laden droplets (particle size): rheological properties of particle suspensions will be investigated. Viscosity and viscoelasticity will be considered as parameters when interpreting coalescence data. Frequency dependent gelation of particle suspensions may prove to be insightful for such coalescing systems.
Particle-stabilized droplets (particle wettability): research will focus on the particle-liquid interface. Particles will be hydrophobically modified using silanation. Once hydrophobic, particles will be dispersed in the organic phase and adsorbed at the droplet liquid-liquid interface. Particle size, concentration, and wettability will be considered as experimental variables. The role of interfacial particles on capillary wave propagation will be elucidated. The mechanism of coalescence will be related to interfacial rheological properties such as; viscoelasticity and yield strength.
It is hoped that the continued studies will provide a greater understanding of coalescence kinetics of particle-laden/stabilized droplets. Previous coalescence models will be adapted to include consideration of bulk and interfacial rheological properties.