57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI548780-DNI5: Near-Field Vibrational Spectroscopy and Imaging of Chemical Species on Individual Nanoparticles During Catalytic (De)Hydrogenation
Michael J. Gordon, PhD, University of California (Santa Barbara)
1. Introduction
The goals of this PRF DNI project are to (1) develop a hybrid scanning probe microscopy system which combines atomic force imaging with near-field vibrational spectroscopy for local identification of surface chemistry, (2) realize catalytically-active nanoparticles with controlled shapes and surface compositions for selective hydrogenaiton, and (3) develop microplasma-based techniques to realize nanostructured metal and metal-oxide films for catalytic and solar cell applications.
During the third (extension) year of the project, grant funds were used to support one PhD student and purchase equipment for microscope development and catalysis experiments. The project has seen considerable advances in year three: (1) all optical, chemical imaging of surfaces at 6 nm spatial resolution has been demonstrated, (2) bimetallic nanoparticle catalysts made of immiscible materials (PtAg) have been developed, and reactivity testing shows that stable surface compositions can be realized to realize both high selectivity and high turnover (in contrast to the common reactivity-selectivity tradeoff encountered in hydrogenation reactions) for partial hydrogenation of C2H2 to C2H4, and (3) the novel microplasma-based deposition method developed in years1-2 has been extended to realize a wide variety of nanostructured metal and oxide materials.
Research directly associated with this work has resulted in 3 publications in 2012 in Nanotechnology, J. Crystal Growth (in press), and Thin Solid Films (submitted). Ancillary work associated with the project has also resulted in 4 other publications in Appl. Phys. Lett., Rev. Sci. Inst., and J. Phys. Chem. C..
2. Experimental Results
(a) Chemical imaging of surfaces
We
have developed and built two hybrid microscopy instruments which
combine scanning probe microscopy,
near-field plasmonic coupling, and Raman spectroscopy to chemically
interrogate
and image materials at length scales that
count to make connections between structure and function. Both
instruments
are currently under extended development with applications in chemical
imaging of
organic solar cell films, catalysts, and biomolecules. Key
results
of the work have been (i) demonstration
of all-optical chemical imaging of
surfaces with spatial resolutions < 6 nm (see Fig. 1),
(ii) rigorous measurement of the distance scaling of plasmonic
enhancements in
Raman scattering (e.g., this work showed that many tip-enhanced
spectroscopy
experiments are plagued by “artifacts,” rather than true tip-surface
plasmonic
coupling), and (iii)
extensive discussion on the design, development, and validation of a
confocal,
side-on tip-enhanced Raman spectroscopy system for chemical imaging of
surfaces
at nm-length scales.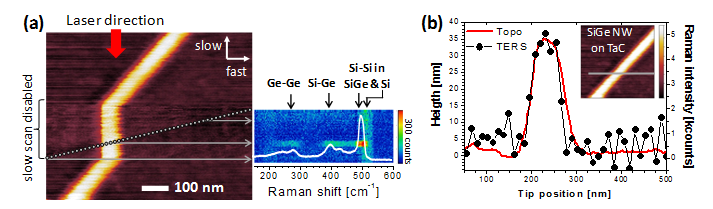 Fig.
1. (a) AFM and near-field Raman imaging of a SiGe nanowire on
TaC. (b) Topography and optical (TERS) profiles of the nanowire,
showing optical resolution below 6 nm.
Fig.
1. (a) AFM and near-field Raman imaging of a SiGe nanowire on
TaC. (b) Topography and optical (TERS) profiles of the nanowire,
showing optical resolution below 6 nm.
(b)
Selective
hydrogenation of alkynes with bimetallic nanoparticles
In
this area, we seek to understand how controlling the surface structure
of
bimetallic nanoparticle catalysts can be used to promote unique
reactivity (e.g.,
high turnover and selectivity simultaneously)
for important chemical reactions (e.g., partial hydrogenation of
alkynes). See
Fig.
2 for details. By
creating nanoparticles from metals that are immiscible
in the bulk, catalysts with unique, more stable surface compositions
and larger
operating windows can be realized. For
example, PtAg nanoparticles with different shapes (octahedra, cubes,
etc.) and
levels of surface Ag-doping were created using colloidal techniques for
selective hydrogenation of C2H2. Catalytic
testing of these
materials, along with PdAg analogues (the common industrial catalyst),
reveals
that (i) the immiscible PtAg system is both highly active and selective
over a
wide temperature window (e.g., Pt with 0.5 monolayer Ag are highly
selective
for partial C2H2 hydrogenation from 175-300
°C at reaction rates comparable
to total hydrogenation on pure Pt), (ii) the miscible PdAg system, due
to
temperature-induced surface rearrangements, becomes non-selective at
high
operating temperatures, and (iii) Ag preferentially decorates
high-energy step/edge
sites on Pt nanoparticles, changing adsorption energies for C2H2
and C2H4 differently, leading to selective
hydrogenation.
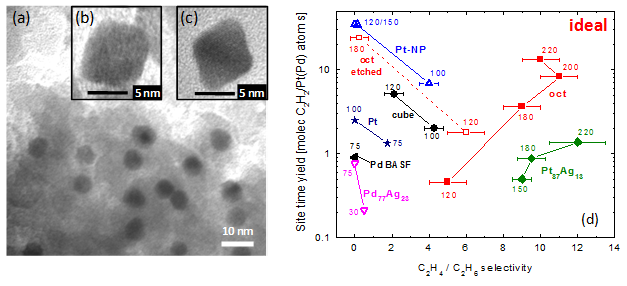 Fig.
2. (a-c) TEM images of PtAg nanoparticle catalysts. (d)
Reactivity testing of bimetallic and traditional catalysts. Turnover
rate and selectivity for C2H2-to-C2H4
at various temperatures are shown.
Fig.
2. (a-c) TEM images of PtAg nanoparticle catalysts. (d)
Reactivity testing of bimetallic and traditional catalysts. Turnover
rate and selectivity for C2H2-to-C2H4
at various temperatures are shown.
(c)
Microplasma-based
synthesis of metal(oxide) nanostructures
Our lab is investigating
plasma-based routes for direct synthesis of nanoparticles and
hierarchically-ordered/structured thin films and nanostructures which
have useful optical, electronic, and catalytic properties. In
particular, we have developed a hydrodynamically-stabilized,
microplasma jet-based growth technique to realize a variety of metal
oxide nanowires (e.g., CuO, PdO, NiO, Fe2O3, SnO2)
on
different substrates (e.g., Si and ITO) at high pressures (10-100
torr). See Fig. 3. Although many examples of nanowire growth using the
vapor-liquid-solid (VLS) method with a catalyst particle exist, our
work demonstrates that anisotropic growth can be realized without a
catalyst, mask, or surfactant using microplasmas to create a directed,
tunable flux of atoms, metastables, and clusters (i.e., by controlling
ballistic vs. diffusional aggregation phenomena) for anisotropic
growth. Variants of the microplasma technique are currently being used
to synthesize porous and textured metal and alloy films as well as
nano- and microstructured oxides for electrocatalysis, gas sensing,
and solar cells.
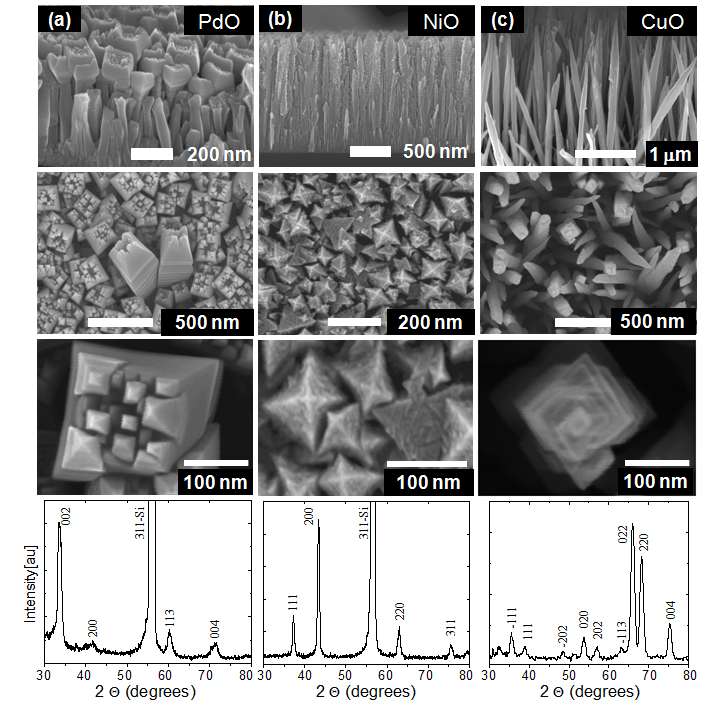 Fig.
3. Various nanostructured metal oxide films formed using
microplasma-assisted, reactive chemical vapor deposition.
Fig.
3. Various nanostructured metal oxide films formed using
microplasma-assisted, reactive chemical vapor deposition.
3. Impact of research
Given the project's focus on theoretical and experimental aspects from nanoscience, optics, and chemical synthesis, the graduate students funded by the project are becoming experts in the fields of scanning probe microscopy, materials characterization, and catalysis. These students work in a unique laboratory setting that provides interdisciplinary training, mentoring, and interactions.
The PRF-DNI grant has also had a substantial impact on the PI’s ability to acquire additional funding. Preliminary results made possible by this grant were incorporated into a successful research grant from the Packard Foundation.
The equipment development/experimental work during the last year has
shown that nanoscale
chemical
imaging of catalytically-relevant surfaces and systems is possible;
in particular, the hybrid optical/scanning probe microscopy system,
synthesis work, and catalytic testing supported by this PRF grant will
allow us to probe and better understand how local effects
influence chemical reactions and molecular transformations on surfaces.










