57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI150330-DNI1: Arylalkoxylation of Alkynes and Olefins via Transition Metal-Catalyzed C-O Activation
Mary P. Watson, PhD, University of Delaware
With generous support from the PRF, we have continued to pursue transition metal-catalyzed activation of C–O bonds to enable rapid generation of molecular and stereochemical complexity from simple, readily accessible starting materials. Our efforts in the activation of strong C–O bonds have led to the development of the first Heck reaction of aryl pivalates. This reaction, developed by graduate students Andrew Ehle and Qi Zhou, represents one of the first examples of C–C bond formation via activation of a strong C–O bond with a non-organometallic coupling partner. It enables the efficient transformation of widely available and inexpensive phenol-based starting materials into styrenyl products without the necessity of expensive triflating agents or formation of triflic acid as a byproduct. This process is highly functional group tolerant, and substrates with nitrile, fluoro, ether, silyl ether, amino, acetal, and heteroaromatic groups can be used. Further, vinyl pivalates can be cross coupled under these reaction conditions, as can a-olefins. Although we were initially surprised that bidentate phosphine ligands provide more reactive catalysts than monodentate phosphines in this reaction, we now hypothesize that bidentate phosphines, such as dppf, may promote a cationic Heck mechanism and thereby facilitate the challenging migratory insertion step. These results have been published and the publication added to the ACS-PRF web reporting system. This publication was one of the top 10 most read articles in Organic Letters from January–March 2012. We are excited to now pursue other reactions via activation of strong C–O bonds. The results of these studies will be reported in due course.
Our group has also begun to develop transition metal-catalyzed reactions of substrates with weaker C–O bonds and has found this to be a particularly interesting area for the discovery of new reaction technology. As we reported in our previous annual report, we developed an enantioselective copper-catalyzed alkynylation of isochroman acetals. During this funding period, we were invited to write a perspective article in Synlett, describing our contributions within the broader context of the field.
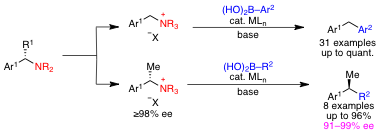
More recently, we have begun to develop transition metal-catalyzed cross couplings via activation of C–N bonds. In this area, we focused on developing stereospecific couplings, because chiral amines can be readily prepared in exceptional enantiopurity. This project was developed by graduate student Danielle Shacklady-McAtee and postdoctoral fellow Prantik Maity. We have just submitted our first publication on this area, which outlines the nickel-catalyzed coupling of benzylic ammonium salts with boronic acids. These reactions proceed under mild reaction conditions and with exceptional functional group tolerance. Further, this method enables transformation of branched benzylic ammonium salts to diarylethanes with excellent chirality transfer, offering a new strategy for the synthesis of highly enantioenriched diarylethanes. To our knowledge, this is the first stereospecific coupling of a benzylic electrophile with a boronic acid and the first coupling of a benzylic amine derivative with high chirality transfer. We are aggressively pursuing other reactions via C–N bond activation and expect that these will provide useful synthetic methods to the chemistry community.
We are very excited to pursue these research directions and are grateful for the funding provided by the ACS PRF, which allowed us to develop this chemistry.