57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI751030-DNI7: Carboxylic Acids as Latent Initiators of Radical Polymerizations Mediated by Hypervalent Iodine Compounds: Synthesis of Functional Polymers and Unimolecular Micelles for Emulsification of Oils in Water
Nicolay Tsarevsky, PhD, Southern Methodist University
Work during the first year of the project focused on i) systematic studies of exchange of carboxylate ligands at hypervalent iodine centers, ii) synthesis of (hyper)branched polymers via in-situ ligand exchange at hypervalent iodine centers by carboxylate monomers; iii) synthesis of dynamic polymers based on hypervalent iodine compounds. Additionally, we initiated studies related to the possibility to convert any (including polymeric) carboxylic acid into radical able to initiate polymerization as well as studies relevant to the preparation of dynamic crosslinked structures.
i) Exchange of carboxylate ligands at hypervalent iodine centers
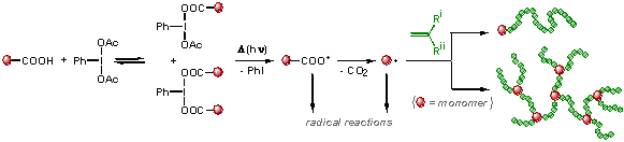
The acetoxy groups in (diacetoxyiodo)arenes, ArI(O2CCH3)2, can exchange with a range of carboxylic acids RCO2H yielding either the mixed carboxylate ArI(O2CCH3)(O2CR) or ArI(O2CR)2 depending on the reagent ratios, the reaction solvent and the temperature. We determined the dependences of the rates and the equilibrium constants of exchange of acetoxy ligands in (diacetoxyiodo)benzene, PhI(OAc)2 (DAIB, Ph = C6H5, Ac = CH3CO2), with methacrylic acid (results published in Polymer Chemistry) and also with 4-vinylbenzoic and acrylic acid (paper in preparation) upon the solvent nature. All obtained hypervalent iodine compounds degrade thermally or photochemically with the generation of radicals, which also contain a polymerizable moiety. In other words, the exchange reactions could be used for the preparation of inimer molecules in situ using inexpensive acidic monomers and DAIB. The inimers can further degrade yielding branched or transiently crosslinked polymers (see below). Additional exchange reactions that are being studied include those between DAIB and bromoacetic, chloroacetic, and trichloroacetic acids. The thermally or photochemically induced homolysis of I-O bonds in the formed hypervalent iodine dicarboxylates is expected to generate the corresponding haloalkylcarboxy or (after decarboxylation) haloalkyl radicals, which can be used either to initiate polymerization (with the formation of polymers with an a-halogen terminus) or to react with carbon nanotubes or fullerenes. Our published and also ongoing studies have demonstrated that virtually any carboxylic acid can be transformed (often in situ) to radicals able to initiate polymerization or useful for other purposes, as shown in Scheme 1. A collaboration has been initiated with Prof. Dieter Cremer (SMU) who will perform quantum chemical calculations that, along with our systematic studies, will help us understand how the structure of the acid and also the reaction medium affect the exchange equilibrium.
Scheme 1. Exchange of acetoxy ligands in DAIB with functional carboxylic acid eventually leading to formation of radicals containing functional groups (including polymerizable moieties)
ii) Synthesis of (hyper)branched polymers via in-situ formation of inimer molecules
A new synthetic method was developed for the preparation of branched polymers that utilizes the exchange of ligands at hypervalent iodine centers with polymerizable acids. For instance, methacrylic acid can exchange with the acetoxy groups in DAIB yielding in situ the hypervalent iodine-based inimers [(acetoxy methacryloyloxy)iodo]benzene (substitution of one acetoxy group) or (dimethacryloyloxyiodo)benzene (substitution of both acetoxy groups). The efficiency of exchange is solvent-dependent. The hypervalent I-O bonds in the inimers are thermally and photochemically labile, and are easily cleaved, generating iodobenzene and the corresponding acyloxy radicals and/or the products of their decarboxylation, which initiate radical polymerization of methyl methacrylate (MMA). In homopolymerizations of MMA thermally initiated by DAIB, i.e., in the absence of the monomer with carboxylic acid group, linear polymers were formed, but in the presence of methacrylic acid, branching and even transient crosslinking was observed. Currently, we are studying the photochemical room temperature polymerization of methacrylates, styrenes and acrylates in the presence of acidic monomers (methacrylic, 4-vinylbenzoic or acrylic acid) in order to demonstrate that the synthetic approach is universal. The prepared hyperbranched polymers will be used as oil dispersants.
iii) Synthesis of dynamic polymers
The I-O bonds in (diacyloxyiodo)arenes are labile and can be cleaved not only homolytically but also heterolytically. It was envisioned that these compounds can be reacted with either dicarboxylic acids (e.g., sebacic acid, dicarboxylate-terminated poly(ethylene oxide), etc.) or polycarboxylic acids (e.g., 1,3,5-benzenetricarboxylic acid, poly(acrylic acid), etc.) and the reaction would yield dynamic linear or crosslinked polymers, respectively. This was proven to be the case. For instance, when sebacic acid was reacted with DAIB (upon heating and application of vacuum to remove the liberated acetic acid), a polymer with dynamic hypervalent iodine-oxygen bonds in the backbone was formed. The polymer reacted with other diacids leading to the corresponding copolymers (Scheme 2, top). When monocarboxylic acids such as acetic acid were added to the polymers, they degraded due to exchange reactions and formation of DAIB. Similarly, poly(acrylic acid) reacted with DAIB in DMF with the formation of gels, which could be degraded upon addition of small amounts of monocarboxylic acids and reformed if the latter was removed (for instance, under vacuum). A publication describing the observed phenomena and their application in the preparation of dynamic polymers is currently in preparation and will be submitted shortly.
Scheme 2. Synthesis of dynamic polymeric materials (linear and crosslinked polymers) via ligand exchange between DAIB and di- or polyacids
We will continue to study the applications in materials synthesis of the straightforward in situ conversion of carboxylic acids (particularly ones with specific functional groups) into radicals. Some of the graft and star-shaped polymers, chiefly those with segmented arms, will be used as unimolecular micelles for oil dispersion.
One graduate student (Mr. Hongzhang Han) was fully supported by the PRF grant. During the first year of the project he employed a variety of analytical and characterization techniques (NMR, SEC, TGA, DSC, viscometry), which were used to study the phenomena and materials described above. He has coauthored a paper published in Polymer Chemistry describing the synthesis of hyperbranched polymers using exchange reactions at hypervalent iodine centers. He just finalized the first draft of a second paper describing the synthesis of dynamic polymers based on hypervalent iodine. In addition, he had the opportunity to give a talk on his research at the 45th ACS DFW Meeting-in-Miniature. Hongzhang Han is interested in working with undergraduate students and has been involved in training one undergraduate student to determine exchange equilibrium constants. These studies will be a part of publication to be submitted shortly.