57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1051497-UNI10: Studies on the Synergistic Effects of Each Component of Hierarchical Ternary Nanocomposites for Lithium-Ion Battery Cathodes
Yuanbing Mao, PhD, University of Texas-Pan American
Advances in energy conversion/storage and related materials are certain to play a central role since our fossil fuel resources are limited, not infinite. As one major energy storage devices, almost all current lithium-ion batteries (LIBs) are explored with single active cathode materials or binary composites, even though optimized nanocomposites and the synergistic effects of each component have pivotal importance. In this study, we are designing and developing hierarchical ternary carbon nanotubes (CNTs)/active cathode V2O5 nanowires/conductive polypyrrole (PPy) nanocomposites for LIB cathodes. It is showing that CNTs provide excellent electrical conductivity, active cathode V2O5 nanowires provide good electrochemical performance, and conductive polypyrrole act as glue between CNTs and V2O5 nanowires to further enhance the electrical conductivity. Moreover, the unique hierarchical architecture provides several advantages. At the meantime, in order to effectively utilize all the desired functions of each component, we are focusing on studying the synthesis-structure-function relationship of the proposed ternary nanocomposites.
During the Year One of this program, two undergraduate students and one M.S. graduate student have worked on the program with interest in pursuing a doctoral degree in the field of physical science and materials engineering. I have trained them on synthesis, surface functionalization, and structural and property characterization of functional nanomaterials. Now one UG has enrolled into the medical school of the University of Texas Health Science Center.
Before making the designed ternary nanocomposites, binary nanocomposites with active cathode V2O5 nanowires/conductive polypyrrole were prepared. Firstly, V2O5 nanowires were synthesized by a hydrothermal process. Specifically, commercial orthorhombic V2O5 powders were added to water followed with addition of H2O2 to form a solution. This solution was then placed in an autoclave, which was maintained at 180°C for 48 h. The resulting precipitates will be collected and purified.
In the next step, we synthesized conductive PPy to glue V2O5 nanowires together by a redox process in aqueous solution. Specifically, it is a two-step process: (1) a oxidant, e.g. iron(III) p-toluenesulfonate (FeTos), is deposited from solution onto the interpenetrating V2O5 nanowire networks by a usual coating method, i.e. solution dip-coating; (2) the formed solution with oxidant coated V2O5 nanowires was then added with pyrrole monomer in an ice cooled beaker. The pyrrole monomer polymerizes on the surface of oxidant coated V2O5 nanowires to form the binary nanocomposites. After the binary nanocomposites, ternary nanocomposites have also been prepared. It involves with in situ synthesis of active cathode V2O5 nanowires to form interpenetrating networks with the functionalized CNTs.
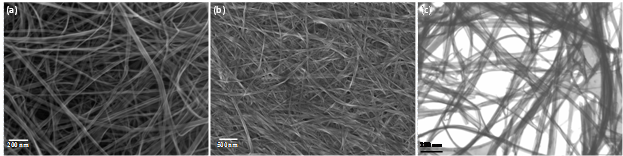
We have chemically and structurally characterized the binary and ternary nanocomposites using different techniques. Chemical and structural information of the synthesized binary and ternary nanocomposites have been collected using X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive X-ray spectroscopy (EDS). XRD, SEM and STEM provide information about the phase, size and shape of the components of the ternary nanocomposites (Figures 1 and 2). EDS will provide information on elemental signatures.
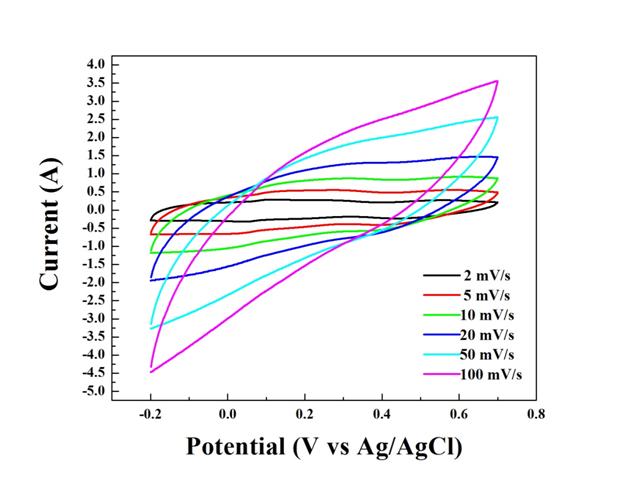
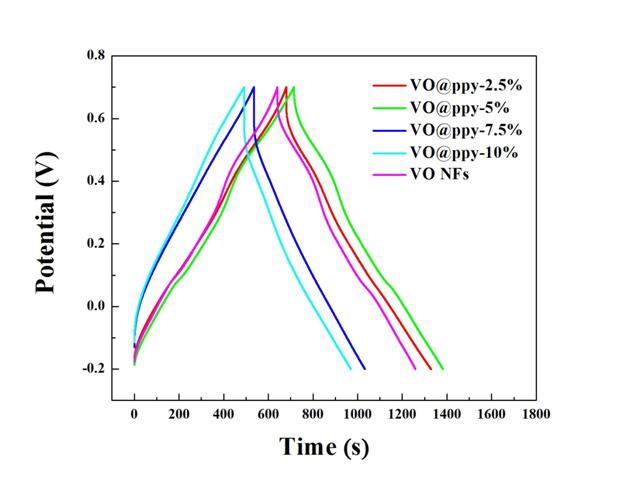
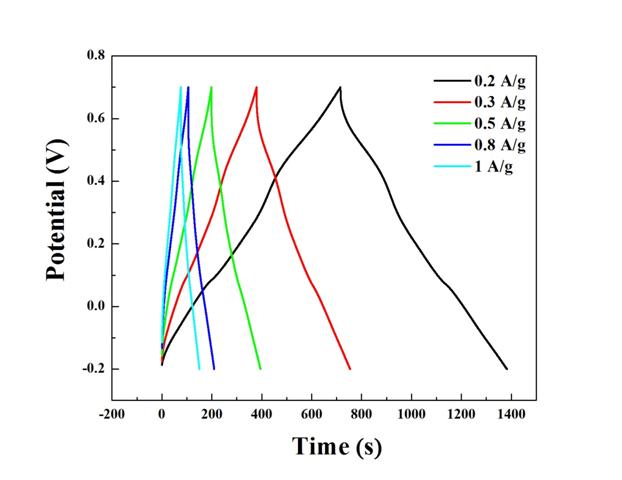
We have also measured the electrochemical properties of the binary and ternary nanocomposites. The information was collected by three-electrode cells to provide preliminary data on electrochemical properties of the binary and ternary nanocomposites. Specifically, the electrochemical properties of the ternary nanocomposites will be measured with beaker-type three-electrode cells. The working electrode is a titanium foil coated with the binary/ternary nanocomposites. The reference and counter electrodes are Ag/AgCl and Pt electrodes, respectively, and the electrolyte is 1M Na2SO4 aqueous solution. Cyclic voltammetry (CV) was carried out at a scanning rate of 2-100 mV/s between -0.2 and 0.7 V (vs. Ag/AgCl) (Figures 3 and 4). Galvanostatic discharge/charge measurements were performed in a potential range of -0.2 and 0.7 V (vs. Ag/AgCl) at different rates (0.2-1.0 A/g) (Figure 5-7). Electrochemical impedance spectroscopy measurements were performed over a frequency range of 100 kHz to 10 mHz at a charged stage with an applied amplitude of 5 mV (Figure 8). This information is correlated with the chemical and structural information.
The graduate student, Elizabeth Martinez and the PI have attended and presented at the Southwest American Chemical Society Meeting held in Austin, TX in November 2011.
Currently, we are making more ternary nanocomposites with various compositions, collecting more data on the ternary nanocomposites, fabricating LIB coin cells, analyzing and correlating the electrochemical data with the structural results in order to identify the synergistic effects of each component in the binary ternary nanocomposites for LIB cathodes. Using the data acquired in prior tasks, electrochemical performance of the ternary nanocomposites based on proper material selection and morphological design and synthesis will be assessed during the second year. This data will be correlated with information from structural and composition characterization. This correlation will be used to determine the synergistic effects of each component for high performance LIB cathodes. We expect this type of nanocomposites will provides a direction toward solving the potential problems of current LIBs and designing the next generation of high-performance LIBs with high electrical conductivity and fast Li+ diffusivity. I also will introduce advanced research ideas into lectures and inspire students' interest and ultimately increase the number of under-represented scientists and engineers.
Figure 1. XRD pattern of V2O5 and V2O5@ppy nanofibers.
Figure 2. SEM images of (a) V2O5 and (b) V2O5@ppy nanofibers. (c) STEM image of V2O5@ppy nanofibers.
Figure 3. Cyclic voltammetry curves of V2O5 coated with different ratios of PPy at the same scan rate: 2 mV/s.
Figure 4. CV curves of V2O5 coated with 5%(wt) PPy at different scan rates: 2, 5, 10, 20, 50, and 100 mV/s.
Figure 5. Charge-discharge curves of V2O5 coated with different ratios of PPy at current density of 0.2 A/g.
Figure 6. Charge-dicharge curves of V2O5 coated with 5%(wt) PPy at different current densities: 0.2, 0.3, 0.5, 0.8, and 1 A/g.
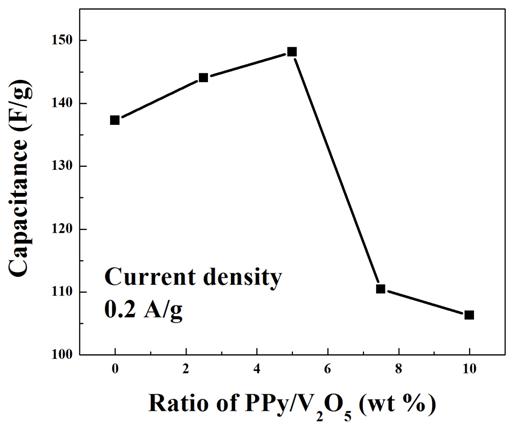
Figure 7. Capacitance vs ratio of PPy/V2O5 (wt%) at current density of 0.2 A/g.
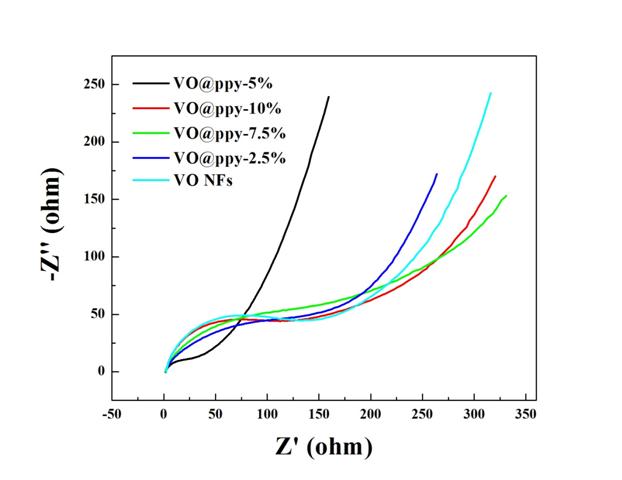
Figure 8. EIS results of V2O5 coated with different ratios of PPy.