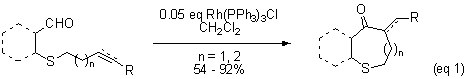57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR149342-UR1: Rhodium(I)-Catalyzed Hydroacylation Promoted by Chelating Amines
Holly D. Bendorf, PhD, Lycoming College
While rhodium-catalyzed heteroatom-directed intermolecular hydroacylation is well documented, relatively few examples of the analogous intramolecular hydroacylation exist.1 Our objective is to apply chelation-assisted hydroacylation to the synthesis of medium ring compounds. Early in our studies, we found that medium-ring sulfur heterocycles could be prepared from alkenal substrates that contain a sulfide capable of coordinating to Wilkinson's catalyst. (eq 1).2
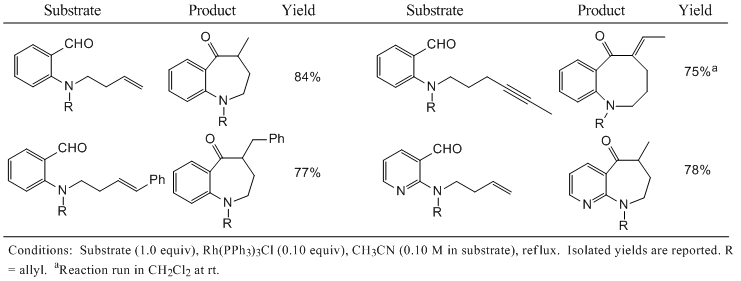
More recent work in our laboratory has sought to adapt the chelation-assisted hydroacylation strategy to the synthesis of medium-ring nitrogen heterocycles, compounds that display a broad range of biological activity. Representative examples of this chemistry are shown in Table 1. 3 Mono- and di-substituted alkenes and alkynes readily undergo hydroacylation to yield benzazepines, however the reaction fails for tri-substituted alkenes. Benzazocines are accessible by the hydroacylation of 4-alkynyl substituted amines and the hydroacylation of alkynes introduces an exo-methylene moiety. Surprisingly, the analogous 4-alkenyl substrates fail to react. The presence of an aromatic amine functionality, such as a pyridine ring, does not interfere with the reaction. The allyl substituents on the amines (represented by R groups in Table 1) do not undergo hydroacylation under the reaction conditions.
Table 1
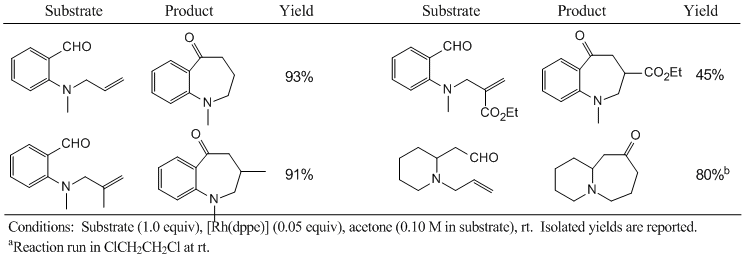
On-going work seeks to expand the utility of this chemistry. Namely, hydroacylation of allyl amines would represent an improvement over the earlier methodology: the installation of the allyl group is straight forward and a variety of substituted allyl bromides, chlorides and alcohols are readily available. Furthermore, this would permit the synthesis of heterocyclic products that do not bear a substituent at the position alpha to the carbonyl.
Treatment of allyl-substituted amines with cationic rhodium catalysts, such as [Rh(dppe)]BF4, yields products due to the hydroacylation of the alkene. Representative examples are shown in Table 2. Hydroacylation of the alkene occurs in high yield for the allyl and 2-methylallyl amine substrates. The presence of an electron-withdrawing substituent on the alkene results in a significantly lower yield of product. At this time, the reaction is fairly limited in scope as internal alkenes fail to react. Ongoing work in our laboratory is directed at optimizing the reaction conditions and choice of catalyst with the goal of expanding the scope of this reaction.
Table 2
Three Lycoming College undergraduates have worked on the project in the past year: Katherine Wellmon, Taylor Hague, and Laura Anderson. Funds from this grant supported two students (KW and LA) during the summer of 2012. Katherine is a senior and is continuing her work on the project. Her research will form the basis of her honors thesis. She plans to attend graduate school next year, with the goal of earning a doctorate in organic chemistry. Laura, a senior, plans to attend medical school. Taylor is a junior and is interested in pursuing a career in the medical field.
1. (a) Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2002, 124, 10296-10297. (b) Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 8078-8079. (c) Takeishi, K.; Sugishima, K.; Sasaki, K.; Tanaka, K. Chem.-Eur. J. 2004, 10, 5681-5688. (d) Coulter, M. M.; Dornan, P. K.; Dong, V. M. J. Am. Chem. Soc. 2009, 131, 6932-6933. (e) Shen, Z.; Dornan, P. K.; Khan, H. A.; Woo, T. K.; Dong, V. M. J. Am. Chem. Soc. 2009, 131, 1077-1091. (f) Shen, Z.; Khan, H. A.; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 2916-2917. (g) Khan, H. A.; Kou, K. G. M.; Dong, V. M. Chem. Sci. 2011, 2, 407-410.
2. Bendorf, H. D.; Colella, C. M.; Dixon, E. C.; Marchetti, M.; Matukonis, A. N.; Musselman, J. D.; Tiley, T. A. Tetrahedron Lett. 2002, 43, 7031-7034.
3. Bendorf, H. D.; Ruhl, K. E.; Shurer, A. J.; Shaffer, J. B.; Duffin, T. O.; LaBarte, T. L; Maddock, M. L.; Wheeler, O. W. Tetrahedron Lett. 2012, 53, 1275-1277.