57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND551503-ND5: Correlating Molecular Environment with Performance in Immobilized Green Catalysts Using Nonlinear Optical Techniques
Julianne M. Gibbs-Davis, PhD, University of Alberta
One strategy for decreasing the energy requirements of catalyzed chemical reactions is to simplifying the purification process. By immobilizing the catalyst onto a macroscopic solid support, separation becomes a simple matter of filtration. Additionally, these immobilized catalysts can often be reused in subsequent reactions. There are numerous examples of such "heterogenized" catalytic systems whereby a well-defined homogeneous catalyst is covalently bound to a support like silica. The types of catalysts vary from metal-ligand complexes to organocatalysts, which lack metals, and have been used in a wide variety of chemical transformations.
Another advantage of immobilizing catalysts on surfaces is that multiple functional catalytic sites can be introduced. These multifunctional catalytic surfaces capitalize on the concept of cooperative or bifunctional catalysis where catalytic groups activate both the electrophile and nucleophile during the course of the reaction. As a result greater activities and enantioselectivities can be achieved. Despite the success of many immobilized systems, an on-going challenge is understanding and predicting whether a certain immobilization strategy will have deleterious effects on the catalytic activity or enantioselectivity of the catalyzed reaction. With our ACS-PRF New Directions grant, we are in the process of applying nonlinear optical methods to the study of well-known immobilized catalysts to ascertain how immobilization influences binding affinity and orientation of the reactant-bound catalyst. In the future we will also examine how solvent influences these parameters, and then determine whether binding affinity, orientation and chiral arrangement correlate with catalyst performance.
Two non-linear optical techniques are being employed in our lab for this project. Thus far the primary technique thus far has been second harmonic generation spectroscopy (SHG), which involves focusing a laser field onto the interface of silica and organic or aqueous solvent. As a result of the symmetry breaking at the interface, new light is generated at twice the incident frequency. When this second harmonic frequency is in resonance with the reactant bound to the surface, the SHG signal increases. Consequently, increases in the magnitude of the second harmonic signal can be correlated directly with the surface coverage of the reactant. The second technique, sum frequency generation is very similar accept that it utilizes resonant enhancement based on excitation of vibrational modes present at the interface. By monitoring the surface coverage of bound reactants as a function of reactant concentration using either technique, the binding constant of the reactant for the surface can be readily determined in real time. Moreover, the magnitude of this second harmonic or sum frequency electric field also changes in predictable ways depending on the polarization of the incident light and the orientation of the reactant. Consequently, information about the orientation and distribution of orientations can also be obtained.
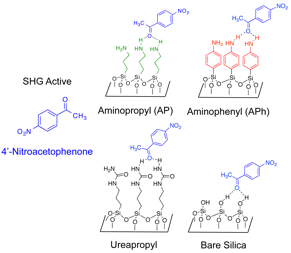
This year we have first focused on exploring a bifunctional organocatalytic immobilized system reported by Lin and Pruski using SHG. In this system, one of the catalytic sites is a urea group, which acts as a Lewis acid and can thereby activate electrophiles like aldehydes or ketones to nucleophilic attack. The other site is a Lewis basic primary amine that can activate nucleophiles like aldol donors and silyl-protected cyanide. To monitor the strength of these catalytic groups interaction with carbonyl containing electrophiles, we sought a methyl ketone that could be resonantly enhanced in the range accessible with our laser system (λSHG = 300-275 nm). We found that 4'-nitroacetophenone was an excellent SHG-active ketone in the required range (λmax = 268 nm). Thus, we monitored the binding of 4-nitro acetophenone in real-time using an incident wavelength of 550 nm and monitoring the corresponding SHG at 275 nm.
Scheme SEQ Scheme 1.
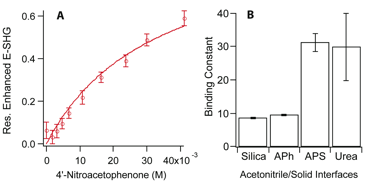
To determine the binding affinity for the silica surface, the ketone concentration was systematically increased, which led to an increase in ESHG (the SH electric field) until the surface was saturated with the ketone and the ESHG began to plateau (Figure 1A). The resonantly enhanced ESHG is directly proportional to the surface coverage. Thus, the Langmuir model can be used to fit ESHG vs [ketone] data and from the fit the Langmuir binding constant can be determined. Figure 1B illustrates the values for the binding constant of the ketone with the silica surface as well as the Lewis acidic urea-modified surface and the Lewis basic aminophenyl modified surface. These values represent the average of two equilibrium constants determined from the fit of two separate isotherm experiments. The binding affinity of the ketone for both the urea and aminophenyl surface were similar suggesting that they were interacting through similar hydrogen-bonding pathways. We conclude that the lower affinity for silica is a result of the weaker hydrogen-bond donating ability of the silica compared with the primary amine or amide.
Figure SEQ Figure 1.
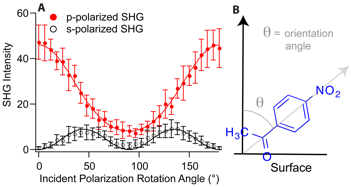
We next turned to polarization-dependent SHG to determine the orientation of the ketone bound to these different interfaces. This can be achieved by monitoring the p-polarized SHG response when the polarization is varied for the incident frequency. By fitting an equation to the data that relates the pertinent tensor elements of the second-order susceptibility of the interface to the signal, the values of these elements can be obtained and related to the average angle between the transition dipole moment of the 4-nitroacetophenone and the surface normal. The table in figure 2B lists the average angles determined for the different interfaces, all of which are approximately 40° with respect to surface normal. This value of 40° corresponds to the so-called magic angle that is obtained when the distribution of angles is very broad regardless of the actual average value. Consequently we infer from our results that all of these surfaces lead to a very broad distribution of angles for the bound reactant. Our future studies are aimed at amine-urea mixed monolayers. We predict that mixed monolayers will lead to optimal binding between the urea and the ketone increasing the binding constant and leading to a different orientational average. These SHG and complementary SFG experiments to determine the hydrogen-bonding environment around the urea groups are on-going.
Figure SEQ Figure 2.