57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1050859-ND10: Colloidal Synthesis of Zinc Phosphide and Tantalum Oxynitride Nanocrystals for Solar Energy Generation
Preston T. Snee, PhD, University of Illinois (Chicago)
The generation of alternative and renewable energy sources is a significant challenge essential towards sustaining long term economic stability and environmental protection. Solar energy collection and H2 production using water splitting photocatalysts are important processes toward achieving our energy goals. However, most photocatalysts can only absorb ultraviolet as opposed to visible light radiation, while visible light-harvesting alternatives suffer from a variety of problems including instability or suboptimal electronic structure for rapid electron transfer. Many light-harvesting systems are also made of highly toxic elements. The results from funding from the PRF have addressed several of these issues; specifically we have developed novel synthetic techniques to create environmentally–benign tantalum nitride, tantalum oxynitride, and zinc phosphide nanocrystals. The tantalum compounds are thought to be catalytically active for water-splitting, while zinc phosphide is an important component in next-generation solar cells. Generally, these materials are synthesized at >1000 °C in vacuum chambers which significantly increases the cost of production. We have developed the methods for semiconductor nanocrystal synthesis and improved upon existing sol-gel methods to produce large quantities of crystalline materials at much lower temperatures at fraction of the cost. The accomplishments of the work supported by the ACS PRF can be summarized as follows:
1) Tantalum nitride (Ta3N5) nanocrystals were synthesized using methods generally employed to create quantum dots; essentially we rapidly inject Ta and N precursors into a ~300 °C solvent and let the nanocrystals grow. While we observed the formation of Ta3N5 nanocrystals, ultimately the reaction yields were found to be very low.
2) As we found that Ta3N5 nanocrystals are prone to oxidation, we decided to examine the formation of tantalum oxynitride (TaOxNy) directly as this material also has catalytic properties. For this study, we developed a sol-gel method that produces large quantities of TaON; furthermore, we demonstrated that the material may be useful for waste-water remediation. Future research may examine solar H2 production from water splitting.
3) Zinc phosphide (Zn3P2) nanocrystals have been synthesized using the rapid injection technique. It was found that previous reports of zinc phosphide nanocrystal synthesis were in error and actually were producing large quantities of metallic zinc nanocrystals. It was also found that our Zn3P2 nanocrystals are highly stable against oxidation in air.
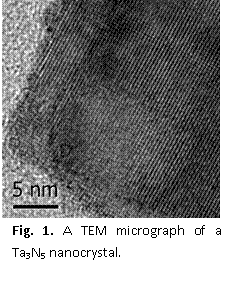
Tantalum nitride nanocrystals were synthesized using three methods as described in our recent publication; an example of an electron microscope image is provided in Fig. 1. This effort was also part of an international collaboration as the Domen group from the University of Tokyo in Japan supplied us with Ta3N5 standards for comparison to our materials. While we synthesized the targeted materials, we found that a substantial fraction of the tantalum oxidized and formed a molecular species before reacting to form the nanocrystal. As such, reaction yields were very low, and we are not able to study the ability of these nanocrystals to split water to form H2 gas.
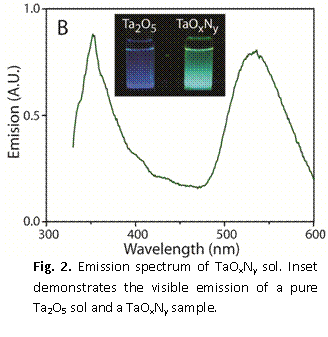
Given the fact that tantalum is prone towards oxidation, we began to study the direct formation of tantalum oxynitride (TaOxNy) via a sol-gel process. The sol–gel chemistry is essentially an inorganic polymerization reaction and is useful for the preparation of inorganic materials such as glasses and ceramics. The first step generally begins with the hydrolysis of a monomeric metal or semimetal alkoxide precursor M(OR)n; in our investigations we used tantalum(V) ethoxide, Ta(OC2H5)5, in a mixed water/alcohol solution. The sol is set to a gel with the addition of an acid or base catalyst. The solvent and low-molecular-weight byproducts are then removed from the system causing a significant level of shrinkage of the gel. While this is a normal route to synthesize tantalum oxide, we were able to dope the material with nitrogen through exposure to ethylene diamine (H2NCH2CH2NH2), although the stoichiometry is not perfectly TaON. Shown in Fig. 2 is the emission of a N-doped sample where a new electronic state emitting at ~550 nm is observed, which demonstrates the success of our doping strategy. Shown in the inset of Fig. 2 are emissions of an undoped Ta2O5 sample (left) and one created with the nitrogen precursor, which indicates that the N-doping of tantalum oxide is visible to the eye. These materials were amorphous, but could be crystallized by annealing at high temperatures; however, this has the effect of lessening the total nitrogen content of the material. We also found that the TaOxNy materials can be used to oxidize model organic waste (nile blue, a common organic dye) in water as discussed in our recent publication. We may measure the catalytic efficiency of this material for solar generation of hydrogen from water in collaboration with faculty at U. C. Davis.
The last project we proposed concerned the synthesis of zinc phosphine (Zn3P2) nanocrystals. For this project we used the method of quantum dot synthesis where diethyl zinc, Zn(CH2CH3)2, and tris-trimethylsilyl phosphine, ((CH3)3Si)3P, are injected into a solvent at ~300 °C. As shown in Fig. 3, we have made small nanocrystals that were confirmed to be composed entirely of Zn3P2. We also repeated the synthesis of these nanocrystals from one other report and found that the dominate material prepared was zinc metal due to a poor choice of a phosphorous precursor. We also found that the material is highly air-stable, which is very unusual as normally zinc phosphide is prone towards reacting with atmospheric water. This makes the material more desirable for use in solar cell applications.
Support from the ACS was instrumental towards developing novel uses for semiconductor nanocrystals regularly synthesized in the labs of the P.I.; several proposals are being prepared based on these works. The preliminary data were taken by a graduate student, Dr. Chiun-Teh Ho, whom is now employed by Intel in Hillsborough , OR. Two other students supported by the PRF are now set to graduate this semester. An undergraduate student, whom is planning to apply to the top chemical engineering graduate schools this semester, is preparing a 1st authored manuscript.