57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI551339-DNI5: Surface Enhanced Raman Spectroscopy of Group IV Semiconductor Electrodeposition and Deoxygenation
Stephen Maldonado, PhD, University of Michigan
OVERVIEW. The overarching thesis of this work is to identify new electrocatalytic methods that produce reduced Group IV elements in order to advance solar energy technologies. We employ a suite of material and surface analytical methods to characterize investigated processes, but rely heavily on Raman as a fast and powerful probe during electrodeposition.
WORK DESCRIPTION. One initial focus of this work was to study the electrodeposition of Ge on Au substrates that support surface-enhanced Raman spectroscopy (SERS) through the controlled electroreduction of GeO2 dissolved in water. We had previously established that this system affords detail on the initial stages of elemental Ge electrodeposition, although the total electrodeposition process was not continuous (i.e. the process self-limits at ~4 monolayers, precluding the possibility of thick Ge films). However, during the course of control experiments, we noticed that the self-limiting aspect was common to all 'standard' electrode materials (e.g. Au, Pt, glassy carbon...etc) but was not similarly operative in electrodepositions performed with amalgams (e.g. Au-Hg). Further, when experiments were performed with liquid Hg as the electrode, the electrodeposition process was unabated over the course of at least a day and yielded mg quantities of Ge. Based on these unexpected results, we assessed the quality and composition of the electrodeposited Ge film. The material was pure Ge (not a Ge-Hg alloy nor a film of hydrogenated Ge) and was crystalline as deposited. Raman and X-ray diffraction analyses indicated the crystallinity was a strong function of the conditions of the electrodeposition and the Ge film was polycrystalline
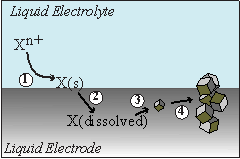
With the support of this program, we have been able to investigate these unexpected results in detail. We have determined that the electrodeposition process for semiconductors like Ge is fundamentally impacted by the solid/liquid nature of the employed electrode. In addition to serving as the source of electrons for the electroreduction of dissolved oxidized precursors, a liquid metal electrode has the added capability of acting also as a solvent in which electrodeposits can partition. If the liquid metal electrode has a finite solubility for the electrodeposited semiconductor, then the liquid metal-dissolved semiconductor solution can reach supersaturation conditions if the electroreduction is allowed to continue long enough. Upon reaching supersaturation conditions, the nucleation and precipitation of the dissolved semiconductor out of the liquid metal will be favored. If conditions are adequate for crystal growth in the liquid metal solution, the net result will be the extrusion of crystalline, fully reduced semiconductors. Figure 1 summarizes this idea which we have dubbed an electrochemical liquid-liquid-solid (ec-LLS) process.
Figure 1. A pictorial representation of the ec-LLS process for the preparation of crystalline semiconductor materials.
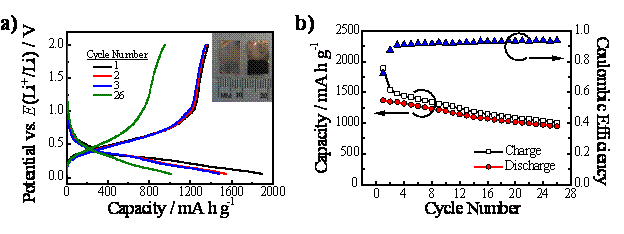
Since this initial recognition of ec-LLS as a new strategy for bulk electrochemical synthesis of Ge, we have begun work on demonstrating the viability of ec-LLS as a means to produce energy technologies without a high energy input, defining the generality of ec-LLS, and understanding the interplay of the steps involved in ec-LLS. In this first funding period, we have made excellent progress in these efforts. First, we demonstrated that ec-LLS in fact does allow the facile fabrication of energy technologies under benchtop conditions (i.e. without excessive heat, exotic solvents, or toxic precursors). We demonstrated specifically that high activity Li+ battery anodes constituted by an electrodeposited crystalline Ge film could be produced through ec-LLS. These results have been recently published in Nano Letters.
Figure 2. (a) First, second, third, and twenty-sixth charge-discharge curves for a Li+ anode recorded at 1C rate using an as-prepared Ge nanowire film electrodeposited onto a Cu support from 0.01 M Na2B4O7(aq) and 0.05 M GeO2(aq). Inset: optical photograph showing a Cu electrode support before and after Ge nanowire film electrodeposition. (b) Galvanostatic Li+ (open squares) charge and (red circles) discharge cycling at 1C rate using an as-prepared Ge nanowire film electrodeposited onto a Cu support from 0.01 M Na2B4O7(aq) and 0.05 M GeO2(aq). (blue triangles) The coulombic efficiencies for each charge-discharge cycle are indicated on the right y-axis.
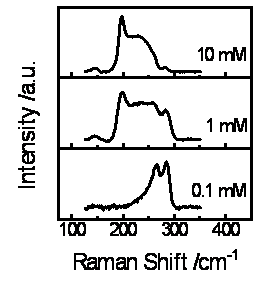
Separately, we have determined that ec-LLS can be exploited for the preparation of crystalline binary semiconductors like GaAs if the liquid metal electrode acts simultaneously as an electron source, a solvent for recrystallization, and a reactant. The electroreduction of a dissolved oxide like As2O3 in water at a liquid Ga electrode has yielded crystalline GaAs under specific conditions. Notably, the use of Raman as a probe to study ec-LLS has proven invaluable, allowing us to better control the resultant product of the electrodeposition. These results have been compiled and submitted for publication.
Figure 3. Raman spectra for films deposited at Ga(l) pool electrodes immersed in an aqueous solution containing 0.1 M NaOH, 0.1 M Na2SO4 at 80 °C with an applied bias of -1.58 V vs. Ag/AgCl as a function of the formal concentration of As2O3.
Figure 4. Optical images of a Ga(l) pool electrode immersed in an aqueous solution containing 0.1 M NaOH, 0.1 M Na2SO4, and 0.001 M As2O3 at 90 °C while biased at -1.58 V vs. Ag/AgCl for 0, 30, 60, 90, and 120 min.
IMPACT OF FUNDS. The support of the ACS PRF program has tremendously benefited my research program. We rapidly advanced the thesis of the work beyond what would have been capable through my original start-up funds. Specifically, I have been able to recruit students for this work with the knowledge that the project has already received some level of external support. To date, we have published/submitted to reports on this work and anticipate submitting two more manuscripts on this project by the end of October, including a major breakthrough on the preparation of Si. Further, my graduate student Junsi Gu has been able to focus his full resources to this research project instead of splitting his time with both research and teaching duties. As a result, he has given formal presentations on his contributions to this project at several professional society meetings (e.g. national meeting of the Electrochemical Society) and to industrial agencies (e.g. Nissan) interested in hiring persons with experience in these technologies.