57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND750863-ND7: Ion Gels from Block Copolymers Composed of Liquid Crystalline Units and Brush-Like Moieties in Ionic Liquids
Rajeswari Kasi, PhD, University of Connecticut
Introduction and general goals
In recent years, the combination of ionic liquid (ILs) and polymer is promising for the development of novel materials because certain polymers are compatible with ILs. The development of polymer electrolytes with unique properties such as thin film formability, flexibility, and transparency in addition to high ionic conductivity has been an intensive topic of many researchers attempting to realize novel electrochemical device. Polymeric ion-gels consisting of a swollen polymeric network in an IL are among the most conductive solid state electrolytes due to their good mechanical properties and high ionic conductivity which can be used in Li-ion batteries, electrochemical devices, sensors, electromechanical actuators, gas separation membranes, and dye-sensitized solar cells. Most of the studies on ion-gels derived from polymers are based on (1) doping of ILs with polymers, (2) in situ polymerization (or cross-linking) of vinyl monomers in ILs. Among them, the utilization of block copolymers is of great interest since this methodology may have the potential to afford easily processable and mechanically strong ion gels by utilizing self-assembly of ABA triblock copolymer in a B-block compatible IL.
It is widely acknowledged that systematic and comprehensive mechanical and electrical property evaluation of ion-gels via self-assembly of block copolymers is desirable. Liquid crystalline block copolymers comprising cholesterol (LC BCPs) are synthesized to harness both the mechanical properties of physically or chemically cross-linked polymer networks as well as the unique features of cholesterol such as chirality, amphiphilicity, and liquid crystallinity. These polymers self-assemble due to supramolecular and van der Waals interactions to form a plethora of interesting and exotic LC structures in the neat- and solution states. We hypothesize that polymers which comprise of LC and brush-like molecules covalently linked through a block-type architecture (LC brush BCPs) will self-assemble in ILs form nano- and mesostructures or hierarchical structures at different length scales. This is due to the interplay of microphase separation, LC characteristics, and solvophobic interactions in ionic liquids, and brush-type architecture and will result in very unique morphologies. To the best of our knowledge, there has been no research effort directed to the synthesis and investigation of the combined influence of LC cholesterol mesophases and brush-like moieties in block copolymer that self-assemble in ILs. Thus the current study focuses on designing highly conductive polymeric ion-gels in ILs through the self-assembly of block copolymer which are promising candidates for solid-state electrolyte applications.
Results and discussions
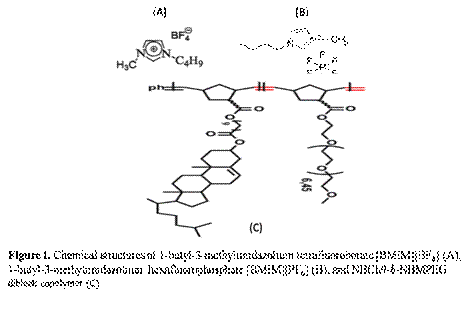
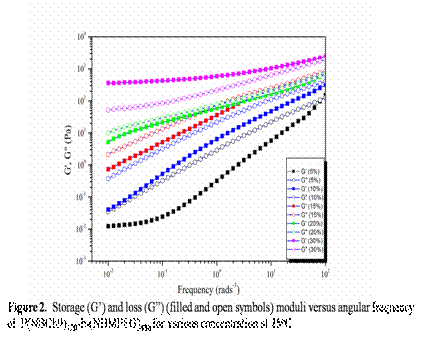
Firstly, we synthesized and characterized a series of random diblock copolymer NBCh9-b-NBMPEG by ring opening metathesis polymerization (ROMP) using modified Grubbs catalyst 2nd generation with CH2Cl2 as a solvent, Figure 1 and Table 1. [BMIM][BF4] and [BMIM][PF6] known as the good solvent for polyethylene oxide block forms ion-gels by non-covalent intermolecular aggregation of the diblock copolymer. Thereafter we investigated the frequency- and concentration dependence of the linear viscoelastic modulus of the diblock copolymer solutions and gels at different concentrations and effect of norbornene cholesterol (NBCh9) block length (or copolymer composition) on the gelation characteristics, Figure 2.
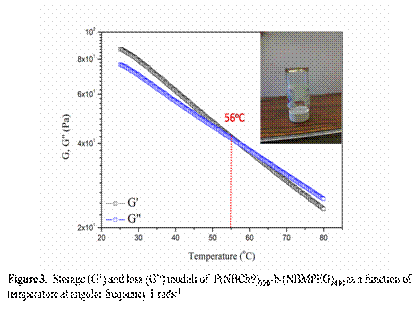
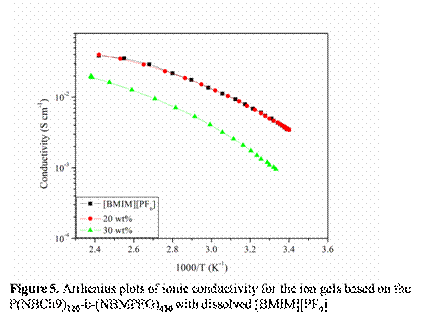
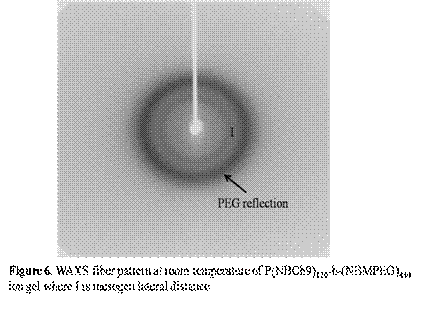
Figure 3 and 4 shows the viscoelastic moduli (G' and G") of the ion gels P(NBCh9)120-b-(NBMPEG)430 as a function of temperature in the range of 20-80oC and compares the elastic moduli of the ion gels when changing the NBCh9 block length. In addition, we investigated the temperature dependent conductivity of the bulk [BMIM][PF6] and the ion gels at different concentrations, Figure 5. Finally, the microstructural analysis of ion-gels is done using WAXS, as shown in Figure 6. The presence of LC mesophases in these ion-gels will help in creating materials with tunable conductivity.
Summary of results
We describe a new set of ion-gels prepared via self-assembly of NBCh9-b-NBMPEG diblock copolymer in a room temperature ionic liquid (IL), 1-butyl-3-methylimidazolium hexafluorophosphate [BMIM][PF6] . At moderately concentrations of 20 wt%, we demonstrate the formation of strong ion-gel with high gelation temperature (Tgel ~ 56 °C) and high ionic conductivities similar to bulk [BMIM][PF6]. It further gives us a handle of copolymer concentration and block length to tune mechanical and conductivity properties so as to meet the design specific criterions for various electrochemical applications. Ionic conductivity studies on these gels have shown moderate affect due to structuring soft materials as compared to bulk IL and thus are good candidates for electrolyte applications. The proposed ionic liquid gels obtained via gelation of [BMIM][PF6] utilizing a brush copolymer is an alternative and attractive way for development of novel solid state electrolytes and offer exciting possibilities of their application in electrochemical devices including Li-ion batteries. Work in progress includes modification of polymer architecture and brush length to optimize mechanical properties and ionic conductivity.
A graduate student was initially funded through this project and a publication based on initial results has been accepted. A postdoctoral fellow has been funded on this grant since Jan 15, 2012. This ND ACS PRF grant opened up a new field of research for the PI, Kasi. We are now able to use a new polymer architecture to prepare materials with inherent mechanical strength and optimal conductivity for electrochemical devices.