57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI651208-UNI6: Insights into the Reaction Mechanism and Catalyst Efficiency of the Conversion of Alkanes to Fuel Alcohols and the Steam Reformation Process
Jonathan T. Lyon, Clayton State University
We are studying reactions between metal atoms and small alkanes as well as metal atoms and fuel alcohols in order to understand the reaction pathways and intermediates that form in the conversion of alkanes to fuel alcohols (i.e., CnH2n+2 + ½ O2 → CnH2n+1OH), and vice versa, on transition metal catalysts. Our investigations combine theoretical techniques to predict the stability of intermediate species and their molecular properties with experiments to synthesize and trap reactive intermediates in an inert argon matrix. Comparison between the two methods (i.e., theory and experiment) will ultimately yield structural assignments of the intermediates in the catalytic conversion between alkanes and alcohols.
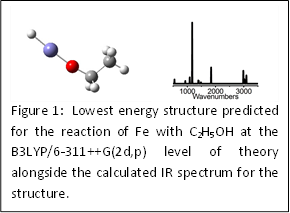 |
Students working under the direction of the principle investigator, Dr. Jonathan T. Lyon, have theoretically modeled the reaction pathway from methanol to synthesis gas (CO + H2) on both an iron atom and a vanadium atom acting as the catalyst. Synthesis gas can be used to make many alkanes and fuel alcohols. Additionally, another student has modeled the interaction of ethanol with an iron atom. Thus, we have been able to compare differences in the reaction pathway for fuel alcohols of differing sizes (i.e., methanol and ethanol) reacting with an iron atom. In all three studies using density functional theory, the lowest energy isomers are found to be the initial insertion products H-M-O-CH3 (M = V, Fe) and H-Fe-O-CH2-CH3. For all species, vibrational frequencies were calculated (Figure 1) and will be compared to our future experimental observations.
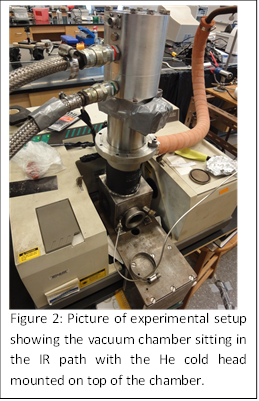
During the past year, work has also been performed to construct the experimental apparatus capable of performing the proposed matrix isolation experiments. This apparatus will allow us to identify the intermediates that form when transition metal atoms are reacted with methanol and ethanol (Figure 2). Metal atoms will be generated by laser ablation using an Nd:YAG laser purchased using funds provided by this PRF award. The metal atoms will be co-deposited with a dilute mixture of fuel alcohols (i.e., methanol and ethanol) in argon onto a ~10 K CsI window, which is cooled by a liquid helium cryostat. Once a large enough concentration accumulates on the window, the intermediates will be observed using infrared spectroscopy. The reaction and deposition is carried out in a vacuum chamber which will be pumped to ~10-6 torr during the experiments. After deposition, the matrix will be exposed to several annealing cycles as well as UV radiation. Infrared spectra will be collected after deposition and after the matrix is exposed to each thermal and/or radiation treatment. Different IR peaks can be grouped and attributed to specific species within the accumulated argon matrix based on the behavior of the absorptions. The experimental frequencies for a given group will be compared to those predicted by theory for several isomers. It is expected that the vibrational structure predicted for the lowest energy isomers will be observed in the experimental spectra, confirming our theoretical predictions.
Select preliminary results of this study were presented by the principle investigator and an undergraduate student (Mr. Marcus Bartlett, Chemistry 2013) at the 2011 Southeastern Regional Meeting of the American Chemical Society (SERMACS) in Richmond, VA. This PRF grant has financially supported two undergraduate students (Mr. Patrick Drew, Chemistry 2012 and Mr. Marcus Bartlett, Chemistry 2013). Additionally, a high school student who was supported by an ACS SEED project award (Ms. Minh-Thu Phan) has participated in the project. All three students have presented their results at least once during the past year to faculty and students on Clayton State University's campus (e.g., at the Department of Natural Sciences end of semester Research Presentations, the university wide Academic Conference, or both). The principle investigator and students are sincerely grateful for the financial support from the ACS Petroleum Research Fund which has aided in establishing a productive research program for undergraduate students at Clayton State University.