57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI451292-UNI4: Using the Photo-Fries Reaction as a Photochemical Probe to Quantify the Cage Effects of Ionic Liquids
Amy E. Keirstead, PhD, University of New England
Introduction. Room-temperature ionic liquids (ILs) are molten salts that are liquids at or below room temperature and have recently attracted considerable attention as environmentally friendly alternatives to conventional (molecular) solvents. Ionic liquids exhibit extremely low volatility, high ionic conductivity, high thermal and chemical stability, and are generally nonflammable and air-stable. The versatility of ionic liquids and their robustness under extreme conditions make them ideal for a wide variety of applications, including solvents for synthesis and catalysis, host materials for molecular electronic and optoelectronic devices, electrolytes in dye-sensitized solar cells (DSSC) and for use in CO2 sequestration. Despite the rising popularity of ILs, the physicochemical properties of these interesting materials have yet to be fully characterized. In this project, the cage effect of ILs is under investigation; the cage effect is a measure of restriction placed on solute molecules by the solvent cage, and describes how effectively the species can escape the solvent cage. Ionic liquids could have large cage effects due to their viscous and ionic nature that could give rise to strong electrostatic interactions with guest species. These cage effects could influence product distributions and/or catalytic activity in IL-solute systems; likewise, the efficiency of the iodide/triiodide redox couple in the IL-containing DSSCs could be dramatically reduced if the IL has a large cage effect. An enhanced understanding of IL cage effects obtained via this study can be combined with other known properties of ILs (such as viscosity and polarity) to allow for the tailoring of ILs to specific applications, including the construction of more efficient DSSCs and the use of green "designer solvents" for chemical processes.
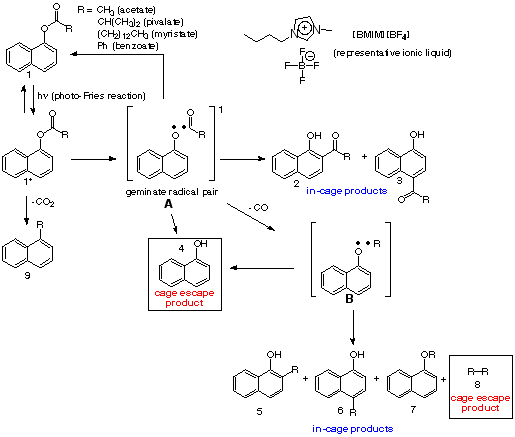
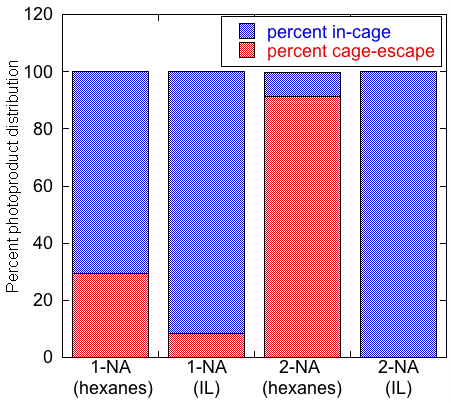
Goals for phase 1 and progress made. The photo-Fries reaction is the photochemical probe reaction used to report on the cage effects of ILs. In this reaction (Scheme 1), the photoexcitation of anaryl ester results in homolytic cleavage of the ester C-O bond to form a radical pair located within the solvent cage. A number of reaction pathways are available to these radicals, where the products can either be classified as in-cage(IC) or cage-escape (CE) products. Gas chromatography-mass spectrometry (GC-MS) is used to identify and quantify the photoproducts and determine the ratio of the yields of all IC and CE products. The preliminary work included in the ACS-PRF-UNI proposal illustrated a positive proof-of-principle, where the ratio of IC:CE products was much higher in the ionic liquid butylmethylimidazolium tetrafluoroborate [BMIM][BF4] than in hexane, suggesting a large cage effect (Figure 1).
The primary goals for the first reporting period were (i) to complete remaining groundwork and address reviewer concerns and (ii) to use this foundation towards generating higher-quality replicates of the preliminary data before (iii) extending to other ester-IL systems to examine how the cage effect is influenced by IL properties (viscosity, polarity) and those of the ester probe (steric/electronic factors). Instrumentation limitations hindered productivity somewhat during this period; the GC-MS used for collection of the preliminary data was out-of-order and unable to be repaired, and off-site product analysis is impossible due to the sensitive nature of the samples. Instead, GC-FID was used to conduct groundwork experiments with the goal of generating higher quality data once GC-MS was available.
Figure 1. Comparison of in-cage and cage escape product yields obtained upon irradiation of 1- and 2-naphthyl acetate in hexanes and the ionic liquid [BMIM][BF4].
The first study addressed a reviewer comment regarding the best irradiation wavelength (UVA vs. UVC) for the photochemical reaction; the former had originally been chosen to preferentially excite the naphthyl ester in the presence of the ionic liquid but a reviewer suggested that UVC irradiation might be more effective. Our results showed that UVC light was indeed more effective and that the ester absorbed so strongly that side-reactions with the IL were not occurring. The ideal irradiation conditions and time to maximize photoconversion and avoid secondary photochemistry using UVC were determined.
The second item examined the procedure for extracting the photoproducts from the ionic liquid; since the ILs are non-volatile, the photoproducts need to be extracted into a volatile organic solvent before injection into the GC. A reviewer suggested that the measured photoproduct distribution might be altered by this process, so a series of control experiments were carried out to address this concern; the results showed that when the extraction was carried out quickly, at room temperature, and in the dark, the photoproduct distribution did not change. As a secondary outcome, the extraction process was streamlined and methods adapted to eliminate contamination e.g., from the rotary evaporator.
A third study aimed to separate the constitutional isomers (3 and 4) and (5 and 6), which previously had eluted as one component, but if separated and quantified, could provide some additional information regarding cage effect and radical recombination. Likewise, the decarbonylation product 9 is known to involve a conformational change that is significantly impeded in rigid media or those with a large cage effect so effort was invested in its identification and quantification. Although considerable data was obtained towards achieving these goals, the results are inconclusive at this stage.
Futurework. The PI of this award was a co-PI on a successful NSF Major Research Instrumentation grant to purchase a state-of-the-art GC-MS to be installed on-site in October 2012, thus future work can proceed as intended without instrument limitations. Experiments will be repeated using groundwork achieved during the first year with an initial focus on concretely identifying all photoproducts as outlined in study 3, above. Additional future work will then proceed as outlined in goals (ii) and (iii), above.
Greater impacts. UNE is a primarily undergraduate institution with a reputation of providing high quality research experiences for its students. Lindsey LaPointe (Chemistry/Secondary Education, 2015) worked on this project during summer 2012. She will continue on the project next spring and summer, continuing to learn hands-on lab techniques and hone her critical thinking, problem solving and communication skills as well as take on a leadership role as additional students are recruited to work on the project.