57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR250759-UR2: Experimental Synthesis of Authigenic Clay Minerals: Implications for Lacustrine Deposition and Diagenesis
Daniel Deocampo, PhD, P.G., Georgia State University
W. Crawford Elliott, PhD, Georgia State University
Project Objectives
The purpose of this project is to test several hypotheses related to the formation and diagenesis of authigenic lacustrine clay minerals using a variety of experimental techniques. Authigenic clay minerals form either from direct precipitation from water or through some interaction between mineral phases and dissolved constituents, perhaps involving biotic processes as well. Many factors are at work in the environment of formation, so interdisciplinary field and lab based studies are required to determine the major controls on mineral precipitation (Deocampo, 2004).
This project is using lab synthesis experiments to test several hypotheses that have been generated in recent years based on detailed studies of clay minerals in saline, alkaline lakes in East Africa and other regions of the world. In these settings, magnesium enrichment observed in ancient lake sediments has been used as an indicator of aridity in ancient times. The recent development of high quality geochronologically controlled stratigraphic sections allows these geochemical indicators to be used in developing high quality paleoenvironmental and paleoclimate change reconstructions (Deocampo et al., 2009).
The hypotheses this project is testing address 1) the role of detrital aluminum-rich substrates on clay authigenisis; 2) the importance of aqueous silica saturation state in determination of clay mineral phase; 3) the effects of carbonate brines on cation exchange (Ca desorption) and clay interstratification; 4) diagenetic clay dissolution due to biogenic CO2; and 5) the importance of alkali concentrations on low-temperature illitization of Fe-reduced smectite.
Experimental
To test hypothesis #1, the hydrothermal sepiolite experiments of Wollast et al. (1968) and Mizutani et al. (1991) have been modified and are being replicated with the additional experimental condition of the addition of an aluminum-rich detrital component (Clay Minerals Society standard clay SWy-2). To test hypothesis #2, synthetic brines are being formulated with varying silica concentrations, and in contact with buffering diatomaceous sediment. To test hypothesis #3, simulated Lake Magadi brines have been formulated (Jones et al., 1977) to test for cation exchange from calcium-saturated standard clays. To test hypothesis #4, experimental clay suspensions are being placed in CO2-enriched waters under ambient conditions, and under high pressure conditions in a Teflon vessel.
Experimental fluids are being monitored by atomic absorption spectrometery. Mineralogy is being monitored with a new Panalytical X-ray diffractometer recently acquired by the project PI with the support of the U.S. National Science Foundation. Geochemistry of 500mg separates is being determined by Rigaku wavelength dispersive X-ray fluorescence spectroscopy, and SEM and TEM facilities will be used once sufficient experimental material is generated to characterize.
Student Participation
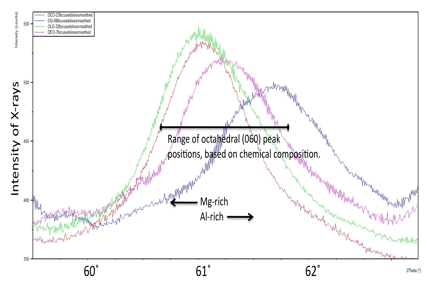
Four undergraduates and one M.S. student have been directly supported by the project, and two additional M.S. students participated in the research while not being supported by ACS/PRF. Four student presentations have been made at national conferences, dealing with the dissolution of clay minerals using the experimental apparatus configured for this project (Huckins and Deocampo, 2012; Raines and Deocampo, 2012a; 2012b), and one by a student who designed the method of CO2 introduction into the experimental brines (Yurman and Deocampo, 2012). The major development in the lab this year was the commissioning of the new X-ray diffractometer, and students have played an important role in the installation and optimization of this instrument, analyzing the various types of geological materials investigated by this project. The XRD has been shown to be very sensitive to the octahedral layer signals so critical to distinguishing magnesium rich versus aluminum rich or iron rich authigenic clay minerals (Figure 1). These analyses were done on ~50mg of submicron (<0.1µm) extracts of clay minerals, representing a substantial improvement in analytical capability.
The team of undergraduates is now engaged under the peer leadership of a MS student with the goal of completing experiments #1, 3, and 5 by the end of 2012, so they may submit their results to present at the southeast section meeting of the Geological Society of America. The team is currently refining experimental procedures to optimize their results in an iterative process, with improving results.
References
Deocampo, D.M., 2004. Authigenic clays in East Africa: Regional trends and paleolimnology at the Plio-Pleistocene boundary, Olduvai Gorge, Tanzania. Journal of Paleolimnology, vol. 31, p. 1-9.
Deocampo, D.M., Cuadros, J., Wing-Dudek, T., Olives, J., and Amouric, M., 2009. Saline lake diagenesis as revealed by coupled mineralogy and geochemistry of multiple ultrafine clay phases: Pliocene Olduvai Gorge, Tanzania. American Journal of Science, vol. 309, p. 834-868.
Huckins, S., and Deocampo, D., 2012. Carbon sequestration in northeast Georgia: potential clay dissolution in the Cambrian Weisner Quartzite (Sandstone). Geological Society of America Abstracts with Programs, vol. 44, p. 62.
Jones, B.F., Eugster, H.P., and Rettig, S.L., 1977. Hydrochemistry of the Lake Magadi basin, Kenya. Geochimica et Cosmochimica Acta, vol. 41, p. 53-72.
Raines, J.E., and Deocampo, D.M., 2012a. Potential mineral dissolution in Black Warrior River Basin caprocks under geological CO2 sequestration conditions. Geological Society of America Abstracts with Programs, vol. 44, p. 62.
Raines, J.E., and Deocampo, D.M., 2012b. Caprock interactions with supercritical Co2 and brine: a laboratory study of the effects of simulated geological CO2 sequestration on shale's from the Black Warrior River Basin, Alabama. Geological Society of America Abstracts with Programs, vol. 44, p. 395.
Mizutani, T., Fukushima, Y., Okada, A., and Kamigaito, O., 1991. Hydrothermal synthesis of sepiolite. Clay Minerals, vol. 26, p. 441-445.
Yurman, S., and Deocampo, D.M., 2012. Carbon sequestration potential in simulated saline lake waters. Geological Society of America Abstracts with Programs, vol. 44, p. 395.
Wollast, R., Mackenzie, F.T., and Bricker, O.P., 1968. Experimental precipitation and genesis of sepiolite at earth-surface conditions. American Mineralogist, vol. 53, p. 1645-1662.