57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI150220-UNI1: Chiral Non-racemic Bicyclic Diketopiperazines: A Common Precursor to Explore Diverse Asymmetric Reactions
Jonathan R. Scheerer, PhD, College of William and Mary
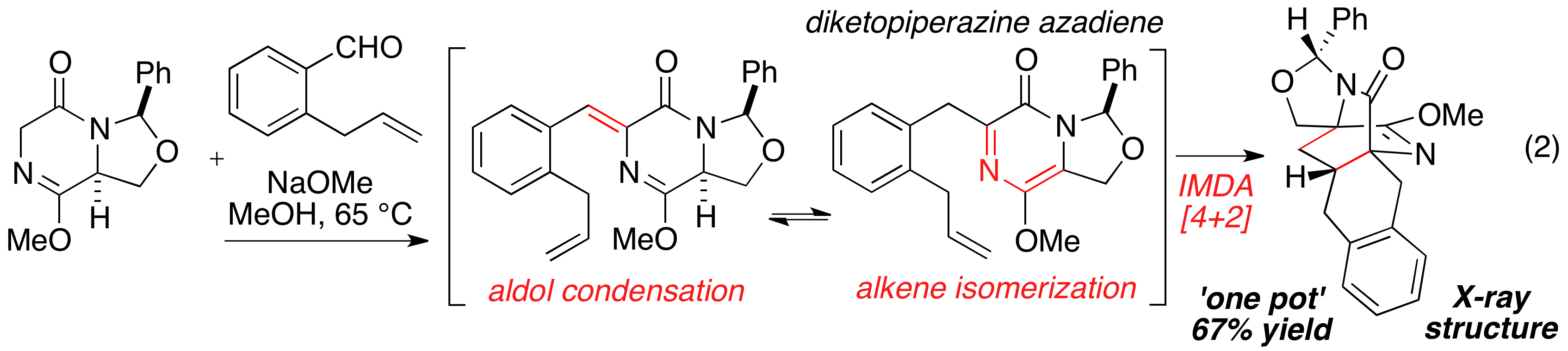
In the second year of funding from the Petroleum Research Fund, we continue to explore the hetero-Diels-Alder cycloaddition with chiral non-racemic diketopiperazine (DKP) azadiene substrates. The key azadiene substrate can be prepared by oxidation of the DKP core (eq 1) or from a multi-component domino reaction sequence involving an aldol condensation, alkene isomerization, and [4+2]-cycloaddition (eq 2). Notably, efficient reaction is observed in all substrates explored to date—both electron rich and electron deficient dieneophiles. Additionally we have determined that 1) the dominant product regioisomer is predictable, 2) the reaction can be performed inter- or intramolecularly, 3) stereochemistry favors reaction from the endo transition state with most substrates, 4) excellent diastereofacial control is enforced with a removable aminal substituent. The chemistry encapsulated in eq 1 and 2 have been extended into two publications.
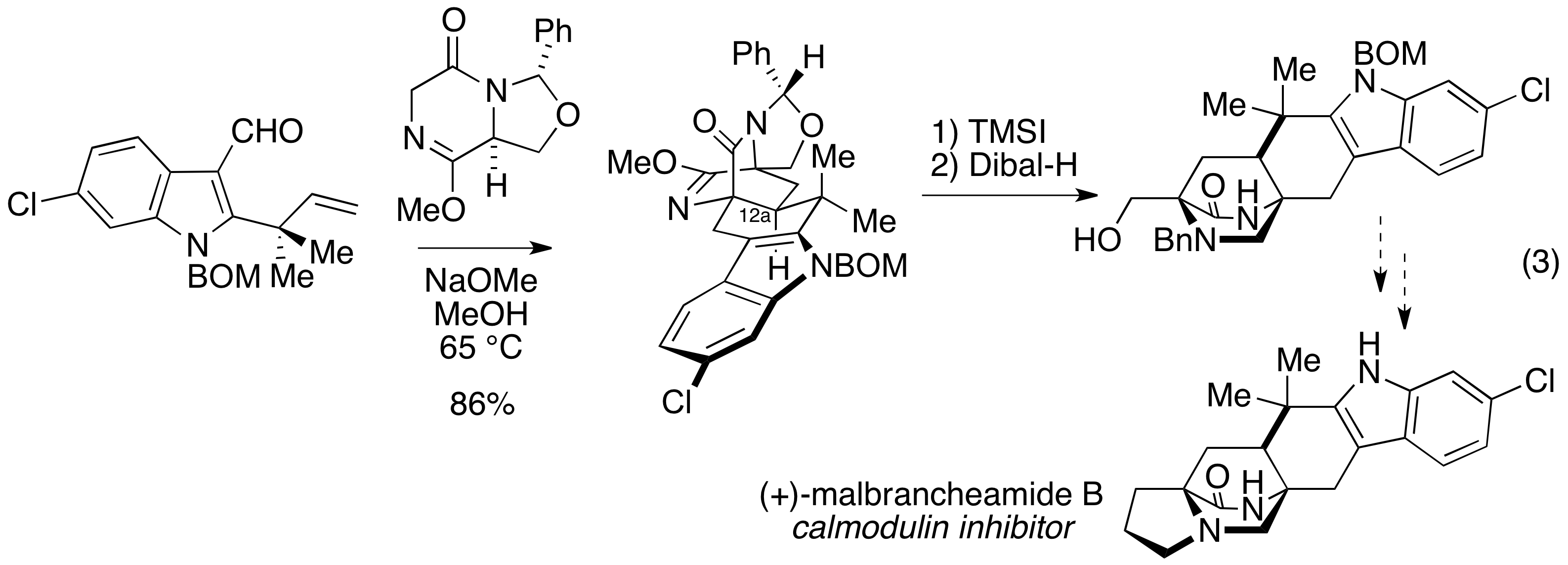
The chemistry that we have explored provides a direct and diastereoselective method to prepare [2.2.2]-diazabicyclic alkaloids. Toward this end, we are using this chemistry to prepare malbrancheamide B, a new calmodulin inhibitor and representative [2.2.2]-diazabicyclic prenylated indole alkaloid. Progress toward this natural product is illustrated in eq 3. We anticipate that completion the synthesis and submission of a manuscript detailing this work will be submitted by the end of the calendar year.