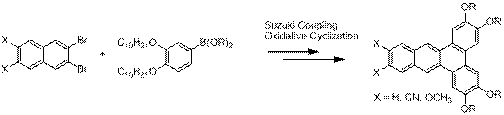57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR1050797-UR10: Synthesis of Novel Polycyclic Aromatic Hydrocarbons Derived from Benzotriphenylene
Kenneth E. Maly, PhD, Wilfrid Laurier University
Polycyclic aromatic hydrocarbons (PAHs) have emerged as a class of organic semiconductors that have potential utility in photovoltaic solar cells, field effect transistors, and light emitting diodes. The semiconducting properties of these materials depend on both molecular properties (such as HOMO and LUMO energy levels) and effective π-overlap between neighboring molecules. Therefore, understanding and controlling molecular organization in PAHs is important for the design of new semiconducting materials.
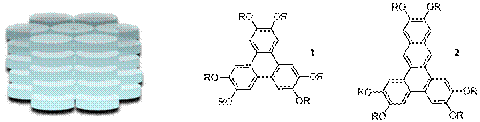
Columnar liquid crystalline phases, where disk-shaped molecules self-assemble into extended π-stacked arrays (Figure 1), are attractive targets for the design of new organic semiconductors. Specifically, the π-π-overlap can facilitate charge transport, while the fluidity of the liquid crystalline phase can be used to prepare aligned films with few defects (which are detrimental to charge transport). The design of new discotic molecules that exhibit columnar liquid crystal phases typically includes a polycyclic aromatic hydrocarbon core with peripheral flexible alkyl chains, such as the hexaalkoxytriphenylenes (1). The corresponding hexaalkoxydibenz[a,c]anthracenes (2) have not been reported, despite the fact that extending the core may promote π-stacking and also lower the HOMO-LUMO gap. One of the primary goals of this research is to prepare a series of dibenzanthracenes and to evaluate their liquid crystalline properties.
Figure 1. Schematic Representation of a hexagonal columnar mesophase, and structures of hexaalkoxytriphenylenes and hexaalkoxydibenz[a,c]anthracenes.
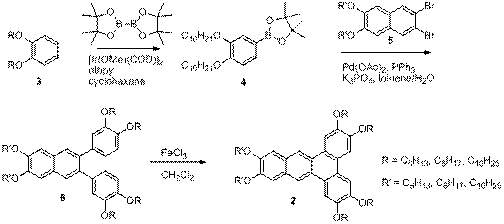
In our lab, we have developed a versatile synthetic approach to hexaalkoxy-substituted dibenz[a,c]anthracenes (1) (also known as benzotriphenylenes) that allows us to explore the effect of structural changes on molecular organization in the solid state and in liquid crystals. The representative synthetic approach for the synthesis of these hexaalkoxydibenzanthracenes is outlined in Scheme 1. A 1,2-dialkoxybenzene (3), which is prepared by alkylation of catechol, is borylated using an iridium-catalyzed direct borylation. The resulting boronate ester (4) is then used in a Suzuki cross-coupling with the corresponding dialkoxydibromonaphthalene (5), to produce compound 6. Compound 6 undergoes an oxidative cyclization to produce the desired dibenzanthracene (2)
Scheme 1. Synthesis of Hexaalkoxydibenz[a,c]anthracenes (2).
Our evaluation of the liquid crystalline properties of these compounds by polarized optical microscopy and differential scanning calorimetry revealed that these compounds did not exhibit any liquid crystalline phases, despite their structural similarities with known liquid crystalline compounds. This result challenges some of the basic design considerations for the preparation of discotic liquid crystalline materials. We hypothesize that the π-stacking required for the columnar liquid crystalline phase is disfavoured in this electron-rich PAH core.
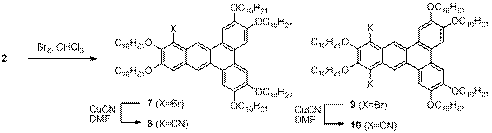
Fortunately, we found that hexaalkoxydibenz[a,c]anthracenes can be regioselectively substituted at the 10- or 10- and 13- positions by electrophilic aromatic substitution (Scheme 2). Furthermore, the halogenated derivatives provide a synthetic handle for further functional group transformations.
Scheme 2. Representative electrophilic aromatic substitution and further modifications of dibenzanthracene 2.
Unlike the parent series 2, all of the substituted derivatives exhibit columnar mesophases, in some cases over very broad temperature ranges, as demonstrated by polarized optical microscopy (Figure 2) and differential scanning calorimetry. The liquid crystalline temperature range is very sensitive to the nature of the substituents. Consistent with expectations, electron-withdrawing substituents induce stable liquid crystalline phases over very broad temperature ranges. However, other factors such as the size of the substituents also appear to affect the mesophase temperature range.
Figure 2. Representative polarized optical micrographs of the hexagonal columnar liquid crystal phase of compounds 7 and 8.
To summarize our progress so far, we have developed a synthesis of a series of novel substituted dibenz[a,c]anthracenes, a class of compounds that has received little attention. Surprisingly, compound 2 was not liquid crystalline; however, the introduction of substituents in the 10- or 10- and 13- positions produces compounds that exhibit columnar liquid crystalline phases. These results highlight the sensitivity of liquid crystalline properties to subtle structural changes, and will guide the design and synthesis of new discotic liquid crystalline materials with tunable properties.
Ongoing efforts focus on expanding the synthetic approach to prepare novel dibenz[a,c]anthracenes with different substitution patterns in order to tune the liquid crystalline properties, as well as the HOMO and LUMO energy levels. By using our synthetic approach that features a Suzuki-coupling and oxidative cyclization (Scheme 3). In addition to the liquid crystalline properties, we will ultimately evaluate the solid-state organization of these compounds by X-Ray crystallography in order to better understand the factors that control molecular self-assembly in this class of compounds.
Scheme 3. General synthetic approach to novel substituted dibenz[a,c]anthracenes.
This grant has directly supported three undergraduate students, who have developed their skills in synthetic chemistry. Two of these students received their Bachelor's degree in 2012 and are currently pursuing graduate studies (one at Western University in Ontario, and one under my supervision at Wilfrid Laurier University). In addition, one Master's student has contributed to aspects of this project. While this student was primarily supported through an Ontario Early Researcher Award, the PRF grant contributed to lab supplies in support of his research. This student completed his Master's in August, 2012, and will be conducting his Ph.D. at Western University in January.