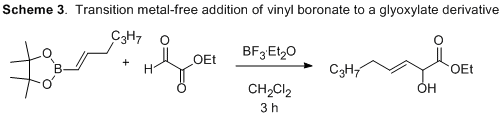57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND150647-ND1: Reactivity of Vinyl Boronates in Enantioselective Ene and Hetero-Ene Reactions
Glenn M. Sammis, PhD, University of British Columbia
Impact of research and results
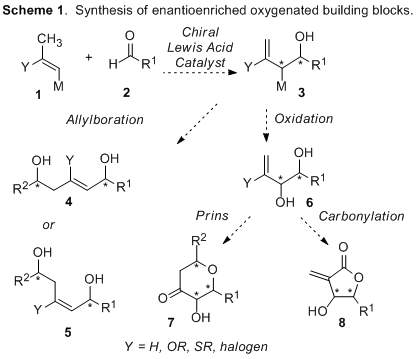
Highly oxygenated small chiral building blocks, such as functionalized 1,5-diols, pyranones, and butenolides, are ubiquitous in bioactive complex natural products and pharmaceutical agents. In addition to being biologically important, these complex fragments present a formidable synthetic challenge, especially in regard to their stereochemical complexity. Tremendous effort has led to many excellent synthetic advances toward the efficient syntheses of these complex motifs. However, their preparation remains a vital area of synthetic research. To address this important problem, we have undertaken a study to examine inter- and intramolecular hetero-ene reactions with 1-substituted vinyl boronates. The boronate-substituted ene products are highly versatile and can be utilized in the synthesis of a diverse set of stereochemically complex building blocks that are common in biologically active compounds (Scheme 1).
For the past year, we have been addressing the first goal of the project that investigates the development of an enantioselective hetero-ene reaction using 1-substituted vinyl boronates. As the literature procedures for the syntheses of the vinyl boronate precursors were either not sufficiently general or did not provide the purity of products we needed in our study, we began by optimizing the syntheses of starting materials to obtain a facile and general route to our desired compounds. We successfully optimized the literature route to provide ready access to catechol and pinacol trans-1-substituted boronates.
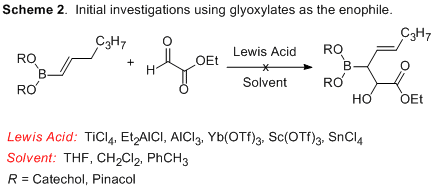
With ample quantities of 1-substituted vinyl boronates in hand, we next investigated their reactivity in hetero-ene reactions with glyoxylate derivatives. Reactions were screened for using a variety of different solvents, temperatures and Lewis acids, encompassing those most commonly employed in the carbonyl-ene reaction such as TiCl4, Et2AlCl, AlCl3, Yb(OTf)3, Sc(OTf)3, and SnCl4 (Scheme 2). Catechol boronates proved too unstable under the reaction conditions, and decomposed prior to reactivity with the glyoxylate. Pinacol boronate-substituted hexane derivatives were stable, but were unreactive at ambient temperature.
When the reaction was catalyzed using BF3 in dichloromethane, consumption of starting material was observed. The observed product was not the anticipated hetero-ene product, but instead was formed from the direct addition of the vinyl boronate into the aldehyde; a reaction pathway usually only observed in the presence of a transition metal. Thus, this intriguing reactivity has the potential to serve as the basis of a new enantioselective Lewis acid-mediated transformation. In addition to investigating this direct addition product, we are also currently investigating derivatives with activated allylic positions to help promote the desired hetero-ene pathway.
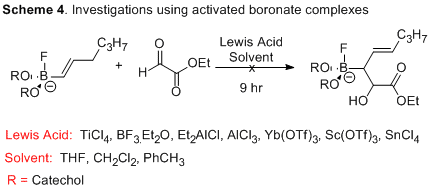
Investigations were then focused on employing a boronic ester anion, which may promote carbonyl-ene reactivity. The addition of a base should increase the electron density at the boron, making the ene component less π-acidic. As before, reactions were screened for employing different solvents, temperatures and Lewis acids. Unfortunately, no reactivity was observed during these screens. We have also investigated the reaction of catechol and pinacol boronates with simple aldehydes. While there is no reaction at room temperature, some conversion of starting material is observed at elevated temperatures. We are currently investigating microwave-assisted hetero-ene reactions.
The low reactivity of the desired intermolecular reaction pathway has prompted us to also begin investigations into our second proposed strategy in which the ene and enophile components are tethered through a boronate ester. Tethering is known to accelerate by enforcing proximity between the ene and enophile component and should promote the desired pathway. We have just started to synthesize the necessary starting materials to test this hypothesis.
Impact of career
This project has had a very beneficial effect on my research career. My research group has been primarily focused on radical chemistry and radical transformations. This project has allowed my research program to expand to investigate ionic methodologies. One drawback of much of the radical chemistry that I have been investigating is that it is not amenable to enantioselective catalysis. This project provides a foothold in this important area and one that we hope to further exploit. I am particularly interested in the reactivity observed between the 1-substituted vinyl boronate and the glyoxylate derivative because this reactivity has the potential to be immediately explored in the context of asymmetric catalysis. Furthermore, the student funding has been invaluable during this early period in my research career.
Impact on student training
This project has been an outstanding training environment for both the graduate student and the undergraduate student who have thus far investigated the reactivity of 1-substituted vinyl boronates. Fundamental to my student mentoring philosophy is providing a training environment where students learn outstanding synthetic technique; careful and consistent experimentation is fundamental to success in graduate school and beyond. Learning how to handle sensitive borane and boronate compounds has been an immeasurably valuable training experience to both my graduate student, and particularly my undergraduate student involved in this project. Furthermore, the investigation of new modes of reactivity is fundamental for the development of any methods chemist. Learning how to come up with new ideas to overcome challenges has been an especially valuable skill. This project has also reinforced the importance of careful product analysis given our fortuitous findings.