57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND351178-ND3: The Role of Protons in Charge Transfer Reactions of Metal Oxide/Solution Interfaces
James M. Mayer, PhD, University of Washington
The ACS-PRF New Direction grant has enabled us to start and pursue a number of projects exploring how protons modulate the interfacial reaction chemistry of zinc oxide and titanium dioxide nanoparticles (NPs). These are very extensively studied systems but the critical role(s) of protons has not received much attention. We are very excited that our first paper supported by this award has appeared in Science, illustrating the potential impact of our continuing studies.
Studies of zinc oxide NPs have received the larger share of our attention because they are very well characterized. These particles are formed by controlled hydrolysis in ethanol/DMSO, capped with dodecylamine and suspended in toluene or toluene/THF mixtures. UV irradiation has long been known to form stable reduced particles, by the excitonic hole (h+) in the valence band oxidizing an organic molecule. We have shown that the irradiation produces 1‑dodecanamine, N‑ethylidene (DDI), by GC, GC-MS, and NMR (eq 1). DDI is formed by oxidation of ethanol remaining from the synthesis to acetaldehyde which then condenses with a dodecylamine capping ligand. The DDI is produced stoichiometrically with the conduction band electrons, indicating that two protons are produced in this photochemical process as well. Thus the charged particles can be represented by H-ZnO/e–CB, H representing the protons and e– the conduction band (CB) electrons.
2h+ + CH3CH2OH + C12H25NH2 ^ 2e–CB + 2H+ + C12H25N=CHCH3 + H2O (1)
These H-ZnO/e– NPs react cleanly with the hydrogen-atom acceptors 2,4,6-tri-tert-butylphenoxyl radical (tBu3ArO●) and 2,2,6,6-tetramethyl-piperidin-1-yl-oxyl (TEMPO), to give the reduced, protonated reagents. The formation of tBu3ArOH and TEMPOH in dry toluene indicates that there has been transfer of e– and H+ from the particles to the organic radical. Titrations have shown the same stoichiometry for reaction of H-ZnO/e–with these H¥ acceptors and with the pure electron acceptor decamethylferrocenium [Cp2*Fe+]BArF4–. Therefore the particles can donate e– or H¥. Similar chemistry has been demonstrated for TiO2/e–, with amorphous NPs, anatase NPs, and slurries of commercial Aeroxide P25 TiO2.
The reaction kinetics between reduced ZnO and TiO2 with TEMPO● or tBu3ArO● have been studied with flash photolysis using ~5 ns pulses of 355 nm light. For both kinds of particles, the UV laser flash causes formation of a small number of conduction band electrons, and presumably surface protons. This is indicated by the low-energy optical absorbance of TiO2/e– and the bleach of the band-edge absorption of ZnO. In the presence of an H-atom acceptor, the signatures of the CB electrons decay over time. The timecourse of this decay indicates, in each case, simple second-order kinetics. The second-order rate constants for the reaction of amorphous TiO2 particles with tBu3ArO¥, for instance, is (1.0 ± 0.4) × 107 M-1 s-1. Mechanistic experiments suggest that this process is a direct hydrogen atom transfer process, but more studies are needed to make this a firm conclusion. We believe that this reactivity is general, that reduced metal oxide surfaces in the presence of protons can act as H¥ donors as well as e– donors.
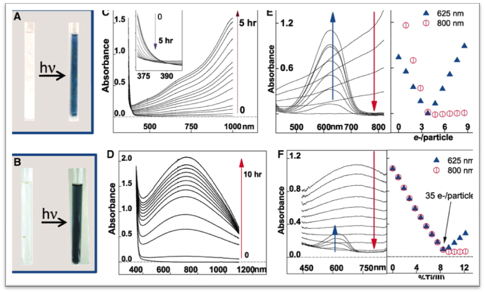
Figure 1. (From Schrauben et al., Science 2012, 336, 1298.) Pictures of as-prepared and reduced (A) ZnO and (B) amorphous TiO2 nanoparticles in toluene. UV-visible spectra of solutions of (C) ZnO (20 mM particles, 3.9 nm diameter) and (D) amorphous TiO2 (51 µM, 3 nm) during irradiation with a 200 W Hg/Xe lamp. tBu3ArO● titrations of (E) ZnO/e– (0.51 mM, 3.9 nm) after 30 minutes of irradiation and (F) TiO2/e– (51 µM, 3 nm) after 10 minutes of irradiation.
These studies are being extended in multiple directions. The kinetics of multiply-charged particles with hydrogen atom acceptors is being examined with a stopped-flow instrument. These reactions do not obey simple second order kinetics, but rather are multiexponential. We are examining how the distribution of rates changes with the number of electrons, from 2 to 40 electrons per particle. Connecting these experiments with the laser-flash studies under different conditions remains an ongoing effort. For ZnO NPs, added dodecylamine slows the rate of H-atom transfer, suggesting that accessibility of surface sites plays a significant role. The distribution and dynamics of the capping ligands are being examined by NMR spectroscopies.
We have discovered that the ZnO NPs can be charged with chemical reductants in toluene/THF solution. While sodium-based reductants were incompatible with the particles (sodium metal, sodium naphthalenide, and sodium anthracenide), addition of decamethylcobaltocene (Cp*2Co) generates reduced particles. The optical and EPR spectra of these charged particles are essentially the same as the photochemically charged ones, even though these have a Cp*2Co+ counterion rather than a proton. Using optical spectroscopy, the extent of reduction of the NPs has been monitored as a function of the amount of reductant added. The maximum number of electrons that can be donated to the NPs range from 1-3 electrons. We are currently studying this maximum number as a function of the capping group and counterions present (protons vs. Cp*2Co+).
All of these experiments are building toward an understanding of how protons and electrons modulate and participate in the reactivity of the metal-oxide surface. We are excited that our new approach is providing interesting and valuable new insights in this important area. Some of these results provided the basis for my portion of a joint grant proposal to the National Science Foundation in July 2011, which was funded for three years starting in March 2012.