57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND350806-ND3: Development of Unsymmetrical Diboron Compounds for Regioselective Diboration and Chemoselective Cross-Coupling Reactions
Webster L. Santos, PhD, Virginia Polytechnic Institute and State University
As a result of ACS PRF funding, we have been able to generate sufficient preliminary data to be competitive for an NSF proposal that will be submitted in the September deadline. There are two students whose research is being supported by this grant. Steven B. Thorpe graduated in the spring of 2012 with a Ph.D. and is now employed at a local biotech, SphynKx Therapeutics, as a principal scientist. Joseph Calderone, a second year student, is a replacement for Dr. Thorpe and has taken over the research project. As a co-author in the two reported publications, Mr. Calderone's training in synthetic organic chemistry and research will likely result in further publications that will allow him to secure a high powered postdoc position or an industrial job.
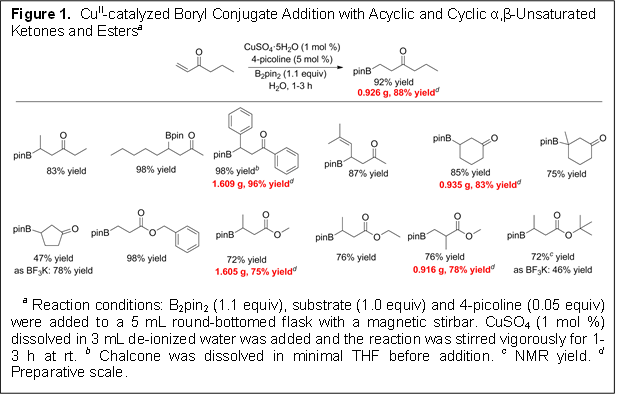
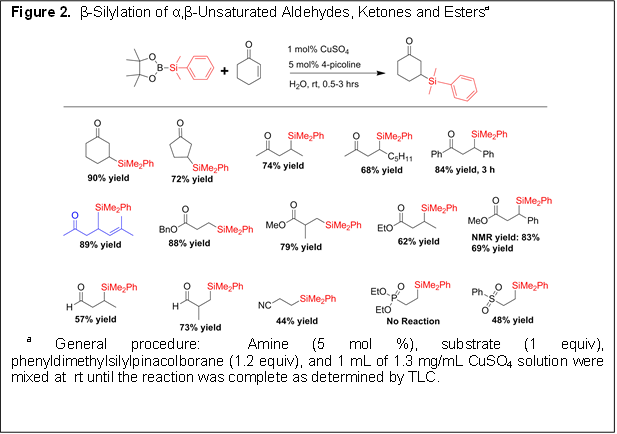
The first year of this grant has been successful and has resulted in two publications. In summary, we have developed user friendly, green and mild methods for the preparation of β-borylated or β-silylated carbonyl compounds. The protocol uses catalytic amounts of copper(II) and amine in water under open atmosphere at room temperature. The scientific impact of the developed procedure is access to compounds as intermediates in the synthesis of complex chemical structures. Further, the mechanistic studies performed will provide knowledge for developing related chemistries.
The major findings during this grant period are as follows:
(1) β-borylation reactions utilizing bis(pinacolato)diboron can be performed in water using a redox stable copper (II) source. This is in sharp contrast with all borylation reactions that use copper as the transition metal catalyst–all use copper (I). As a consequence the reaction can now be performed in open atmosphere–i.e. glovebox or inert atmosphere conditions is not required.
(2) We discovered a rate acceleration of the reaction in the presence of a catalytic amount of amine base. A survey of amine bases present in most laboratories suggests that any monodentate amine will work.
(3) Mechanistic studies suggest that the role of the amine is to deprotonate a nucleophilic water molecule to activate boron and transfer either the silyl or boryl group onto copper.
(4) Redox stable copper sulfate allows the reaction to proceed efficiently in water at room temperature making the reaction green.
(4) The developed reaction in applicable to borylation or silylation of a wide variety of substrates with different functional groups.
Figures 1 and 2 show the conditions and illustrate the substrate scope of the borylation and silylation protocol.