57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1050390-DNI10: Control of Interpenetration in Metal-Organic Frameworks via Spatial Protecting Groups
Ognjen S. Miljanic, PhD, University of Houston
Introduction and Objectives
Metal-organic frameworks (MOFs) are porous crystalline materials created by connecting metal cluster "nodes" through oligofunctional organic "struts" into an infinite framework. High thermal stability, easy synthetic modification, and high porosity have made MOFs promising candidates for applications in gas storage and separation, sensing, environmental remediation, catalysis, and drug delivery. MOFs' porosity and modular synthesis offer unprecedented opportunities for translating properties of functional small molecules from solution to the solid-state, while avoiding common problems associated with this transition, such as the effects of aggregation in the solid state. With our interest in site-isolation chemistry and fluorescent sensing, MOFs present an ideal platform to design, synthesize, and incorporate sophisticated organic linkers into these framework structures and study their structural and functional behavior through a combination of UV/Vis, NMR, and IR spectroscopy, X-ray crystallography, and thermogravimetric analysis.
Results
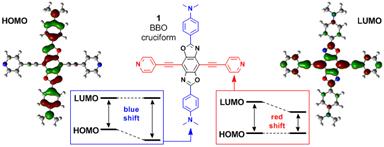
The first major advance we have achieved during this second year of our PRF-funded project was the design and synthesis of benzobisoxazole cruciform-shaped fluorophores such as compound 1 shown in Figure 1. In 1, frontier molecular orbitals—HOMO and LUMO—are localized on different portions of the molecule, meaning that analyte binding always affects only one of these FMOs and not the other. This binding then inevitably leads to a change in the HOMO–LUMO gap and the associated optical properties, and indeed these compounds have been demonstrated to be viable sensors for a variety of different Lewis acidic analytes, including carboxylic and boronic acids, as well as metal ions.
Hybrid fluorescent sensors were built up from cruciform 1 and small non-fluorescent boronic acids; these sensors employed the cruciform as a fluorescent reporter and the boronic acid as a chemically reactive moiety. Thus, reversible reactions of boronic acids with phenols, amines, and small organic and inorganic anions resulted in the change of electronic density on boron and this information was transmitted onto the pyridine nitrogen atom of the cruciform causing a dramatic change in the fluorescence emission. These hybrid sensors were able to qualitatively distinguish among structurally closely related analytes, and such behavior may have potential applications in routine quality control of counterfeited or otherwise compromised pharmaceuticals, alcoholic beverages, and food additives. Because of their rigid geometries and pyridine ligand sites, cruciform 1 and related compounds should be readily incorporated into MOFs and several preliminary structures have been obtained.
Figure 1. Spatial separation of frontier molecular orbitals in the benzobisoxazole cruciform 1 is essential to its use as a sensing component of functionalized MOFs.
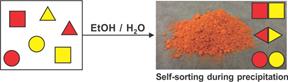
The second direction of our work during the past year focused on the studies of compartmentalization processes, both in the classical heterogeneous sense (physical compartmentalization), as well as in the sense of chemical isolation and non-interference (chemical pseudo-compartmentalization). We are interested in examining the influence that high-barrier MOF encapsulation and other irreversible stimuli exert over complex mixtures of equilibrating compounds. As the model system, equilibrating mixtures of imines were chosen; imines have the general molecular formula X–CH=N–Y and, when multiple imines are present in the same solution, they trade their substituents through an imine metathesis process. These Dynamic Combinatorial Libraries (DCLs) respond to external stimuli by amplifying the component that adopts best to those stimuli. Our previous work has demonstrated that very complex mixture of imines will spontaneously self-sort when exposed to an irreversible stimulus that shows selectivity in removal for certain components of the mixture, and we have already demonstrated that chemical oxidation or physical distillation stimuli can be used to induce this simplification. Recently, we have also demonstrated that simple precipitation of imines will yield similar simplification of complex imine libraries, based on differences in collective solubilities of all possible components of a DCL. We are currently attempting to expand this chemistry to other classes of equilibrating compounds, including industrially relevant esters and alkylated aromatic hydrocarbons. In addition, our future efforts are directing at investigating whether encapsulation within crystals of MOFs with different pore sizes and polarities can be achieved with sufficient selectivity to self-sort a mixture of equilibrating imines. For this purpose, we have developed metal-organic frameworks with long aromatic linkers substituted with multiple fluorine substituents. These MOFs should selectively encapsulate highly lipophilic substrates—at the expense of their hydrophilic counterparts, even in the absences of preferences based on size and/or shape. Our results in this area have been submitted for publication.
Figure 2. Schematic representation of self-sorting that occurs during slow precipitation of imines from ethanol/water solutions.
Impact on the Careers of the PI and Students Involved in the Project
Based on the support from the PRF, the PI has published five additional articles during the second project year. For these and other accomplishments, PI was recognized by the prestigious 2012 National Science Foundation's CAREER award and the University of Houston Teaching Excellence award, as well as invitations to contribute articles and commentaries to prestigious international journals, and present talks at universities in the US and abroad. Jaebum Lim (graduate student) co-authored three of the five articles published with the PRF support; he will graduate at the end of 2012 and has secured a postdoctoral position at the University of Texas. Teng-Hao Chen (graduate student), who was directly supported by the PRF funds, advanced to PhD candidacy in Fall 2011, and is currently working on his second and third publications stemming directly from PRF support.