57th Annual Report on Research 2012 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR351085-UR3: Fundamental Chemistry of the Re(CO)3(H2O)3+ Synthon
Richard Herrick, PhD, College of the Holy Cross
One of the specific goals of this project was to explore new reactions of the triaqua cation, Re(CO)3(H2O)3+, with both traditional and novel ligands. In the first year of this grant, we have made significant progress, looking at the coordination of Schiff base ligands formed by condensation of pyridine-2-carboxaldehyde with 1) aminophenols or 2) phenylenediamines. The ultimate goal is to explore the chemistry of d6 rhenium systems in aerobic, aqueous conditions and to create new materials with novel properties.
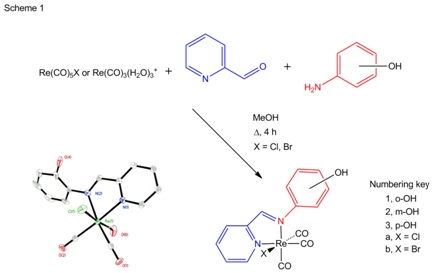
1) Reactions of 2-, 3- and 4-aminophenol with pyridine-2-carboxaldehyde and Re(CO)3(H2O)3+ in one pot reactions gave the expected pyca-C6H4OH (pyca = pyridine-2-carbaldehyde imine) complex in high yield. This was extended to reactions with Re(CO)5Cl (Scheme 1).
This work was spurred by the report that Re(CO)3(pyca-C6H4-p-OH)Cl was prepared and, when treated with base, turns from orange to blue and return to orange on reprotonation [1]. We prepared 5 novel compounds, varying halide and substitution on the phenyl ring. Three new crystal structures, 1a,b and 3b were obtained and all compounds were characterized completely. One characteristic structure, of 2a, is shown in Scheme 1.
A notable discovery was that all o- and p-phenol derivatives deprotonate when exposed to tetramethylammonium hydroxide, producing a blue solution. In contrast, m-phenol derivatives 2a and 2b do not change color when exposed to base. We attribute this behavior to lack of resonance structures that would allow the negative charge to be transferred throughout the ligand. This phenomenon is being explored in more detail, as we study the physical properties of these compounds and the effect of delocalization on properties. We are also extending this chemistry by creating analogous diazabutadiene derivatives, substuting glyoxal for pyridine-2-carboxaldehyde.
2) We also examined the reactions of diimines formed by condensation of phenylenediamines and pyridine-2-carboxaldehyde. In addition to examining the reactivity of the triaqua cation, we are interested in producing extended conjugated structures with bound Re(CO)3+ fragments.
Reaction of chloro or bromo sources of d6 rhenium tricarbonyl with pyridine-2-carboxaldehyde and m- or p-phenylenediamine gave the expected [Re(CO)3X]2(pyca-C6H4-pyca) compounds (the synthesis and spectroscopic properties but not the crystal structure have been reported for the p-phenylenediamine, X = Cl derivative [2].) All four derivatives were prepared and characterized. Neither of the p-phenylenediimine derivatives gave single crystals, however, both of the m-phenylenediimine derivatives were characterized by X-ray crystallography, confirming the expected structure.
An unexpected result was observed in reactions involving o-phenylenediamine. Under a variety of conditions involving either the chloro or bromide reagents, four distinct products were isolated (Scheme 2). Identification of the four products was greatly facilitated following elucidation by X-ray crystallography. Compound 6 is the expected product. Compounds 4 and 5 each feature formation of a pyridine benzimidazole from attack of both amine nitrogens at the carbonyl followed by oxidative loss of H2. Reaction with a second molecule of pyridine-2-carboxaldehyde leads to 5. Compound 7 likely results from formation of 6 followed by oxidative carbon-carbon bond formation in an electrocylclization reaction. The oxidative reactions are likely driven by energy gains from formation of aromatic or conjugated products.
In the second year of funding, we plan to explore new ways of preparing the Re(CO)3(H2O)3+ synthon directly using microwave technology. We believe that this method of preparation will yield several important advantages, including shorter reaction times, higher yields, extensions to halides other than bromide, and aqueous methods of preparing compounds previously prepared in organic solvents.
[1] W. Liu, K. Heinze, Dalton Transactions, 39 (2010) 9554-9564.
[2] P.J. Ball, T.R. Shtoyko, J.A.K. Bauer, W.J. Oldham, W.B. Connick, Binuclear Inorg. Chem., 43 (2004) 622-632.